Isoflavanol compound and preparation method and application thereof
A technology of isoflavanes and compounds, applied in the field of natural phytochemistry, can solve the problems of not being deep enough and unable to identify flavonoids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
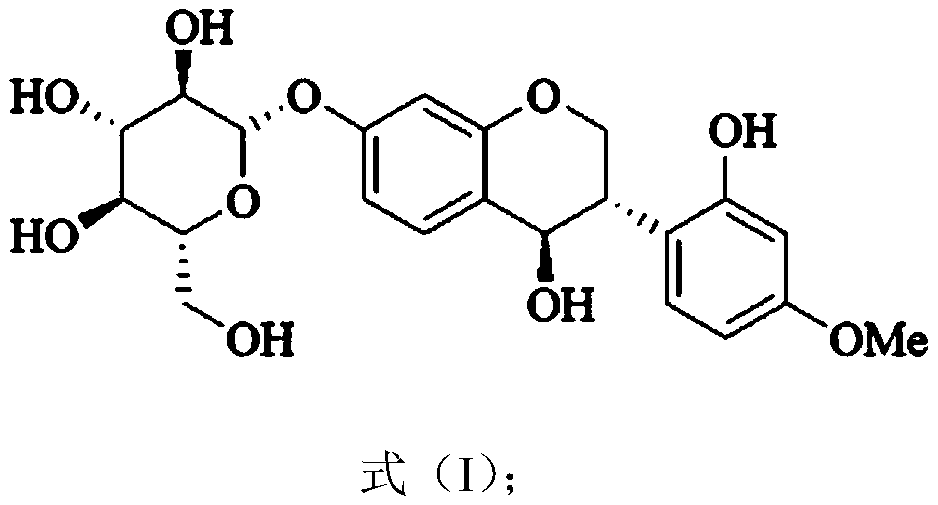
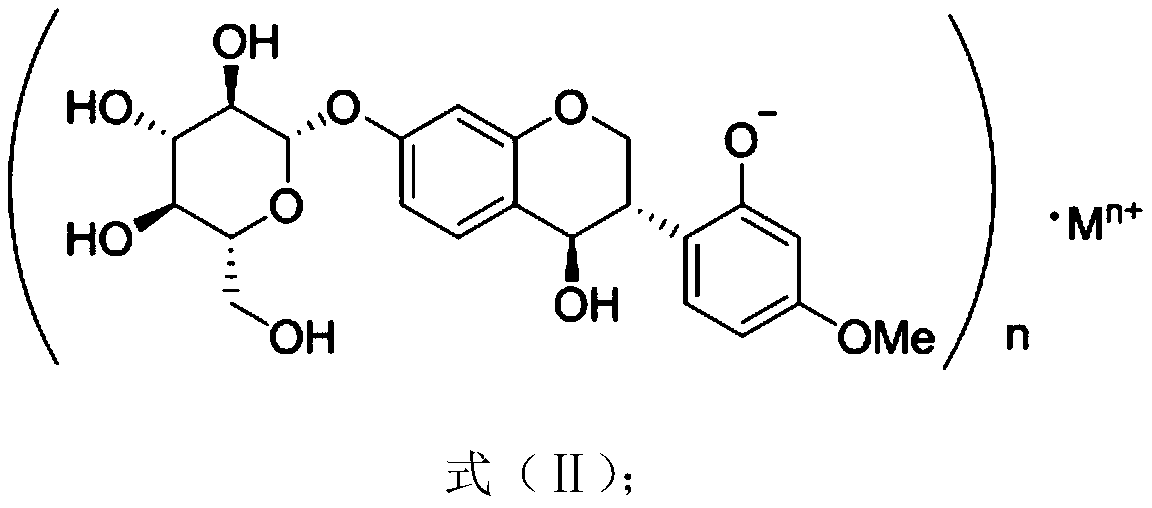
[0036] Example 1 Extraction and separation of compound 4,2'-dihydroxy-4'-methoxyl-7-isoflavanol glucoside
[0037] Genuine medicinal material of Spatholobus spatholobus was collected from Yunnan in March 2016, and was identified as the dried cane of Spatholobus suberectus Dunn., a leguminous plant. The certificate specimen (Y008-1608010) is preserved in the National Key Natural Medicine and Biomimetic Medicine of School of Pharmacy, Peking University laboratory.
[0038] Weigh 70kg of dry Spatholobus medicinal material, add 5 times the amount of water to reflux and extract twice, each time for 2h, combine the extracts, concentrate under reduced pressure, and dry in vacuum at 75°C for 7h to obtain 6kg of Spatholobus dry extract (yield: 8.57 %), keep 1kg, and the remaining 5kg is dissolved with 6L deionized water, extracted 8 times with appropriate amount of ethyl acetate successively, each extract is combined respectively, concentrated under reduced pressure and evaporated to d...
Embodiment 2
[0041] Compound 4,2'-dihydroxy-4'-methoxyl-7-isoflavanol glucoside is in the process of extraction and separation, in S5., Fr.5-2 is subjected to polyamide column chromatography, and can be used for 20: 1 methanol-water solution to obtain the component Fr.5-2-1. Other steps were carried out according to the extraction and separation process described in Example 1 to obtain the compound of the present invention.
Embodiment 4
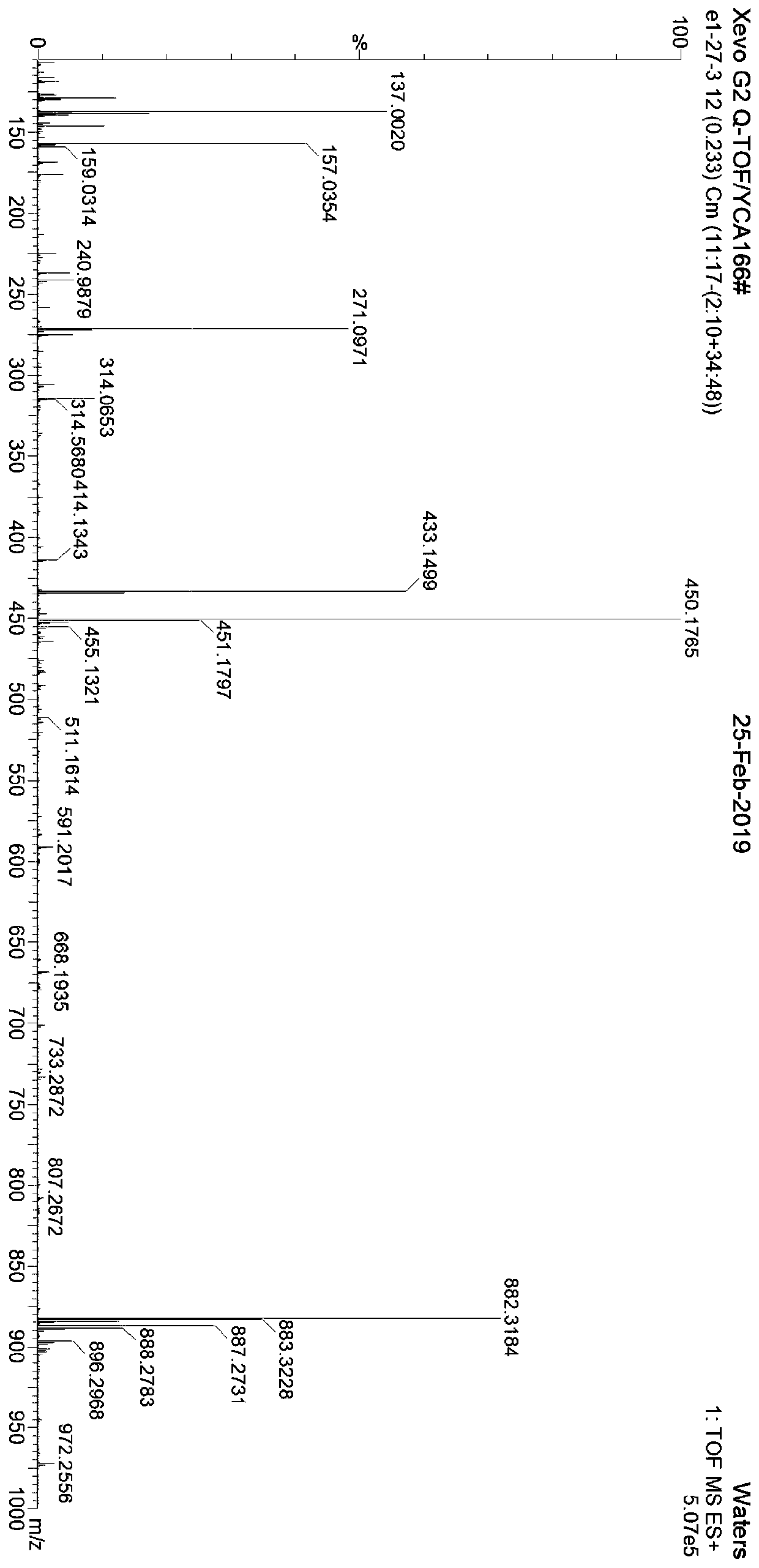
[0042] Example 4 Compound 4,2'-dihydroxy-4'-methoxy-7-isoflavanol glucoside structure identification
[0043] The hydrogen spectrum, carbon spectrum data of described isoflavanol compound are as follows:
[0044] 1 H-NMR (400MHz, DMSO-d 6)δ:3.64(1H,dd,J=9.8,15.9Hz,H-2α),4.29(1H,dd,J=9.2,9.8Hz,H-2β),3.66(1H,ddd,J=6.4,9.2 ,15.9Hz,H-3β),5.61(1H,d,J=6.4Hz,H-4α),7.39(1H,d,J=8.6Hz,H-5),6.72(1H,dd,J=2.2 ,8.6Hz,H-6),6.56(1H,d,J=2.2Hz,H-8),6.43(1H,d,J=2.1Hz,H-3'),6.45(1H,dd,J= 2.1,8.1Hz,H-5'),7.26(1H,d,J=8.1Hz,H-6'),3.70(3H,s,4'-OMe),5.30(1H,d,J=4.9Hz ,2"-OH), 5.08(1H,d,J=4.7Hz,3"-OH),5.01(1H,d,J=5.2Hz,4"-OH),4.55(1H,t,J=5.6 Hz,6"-OH),4.85(1H,d,J=7.4Hz,H-1"),3.23(1H,m,H-2"),3.31(1H,m,H-3"),3.16 (1H,m,H-4"),3.34(1H,m,H-5"),3.44(1H,brdd,J=12.0,5.9Hz,Ha-6"),3.66(1H,brdd,J =12.0,5.7Hz,Hb-6"); 13 C-NMR (100MHz, DMSO-d 6 )δ: 66.1(C-2), 39.9(C-3), 77.9(C-4), 132.1(C-5), 110.6(C-6), 158.6(C-7), 104.2(C-8 ),156.4(C-9),114.3(C-10),119.4(C-1'),160.4(C-2'),96.5(C-3'),160.7(C-4'),106.3( ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com