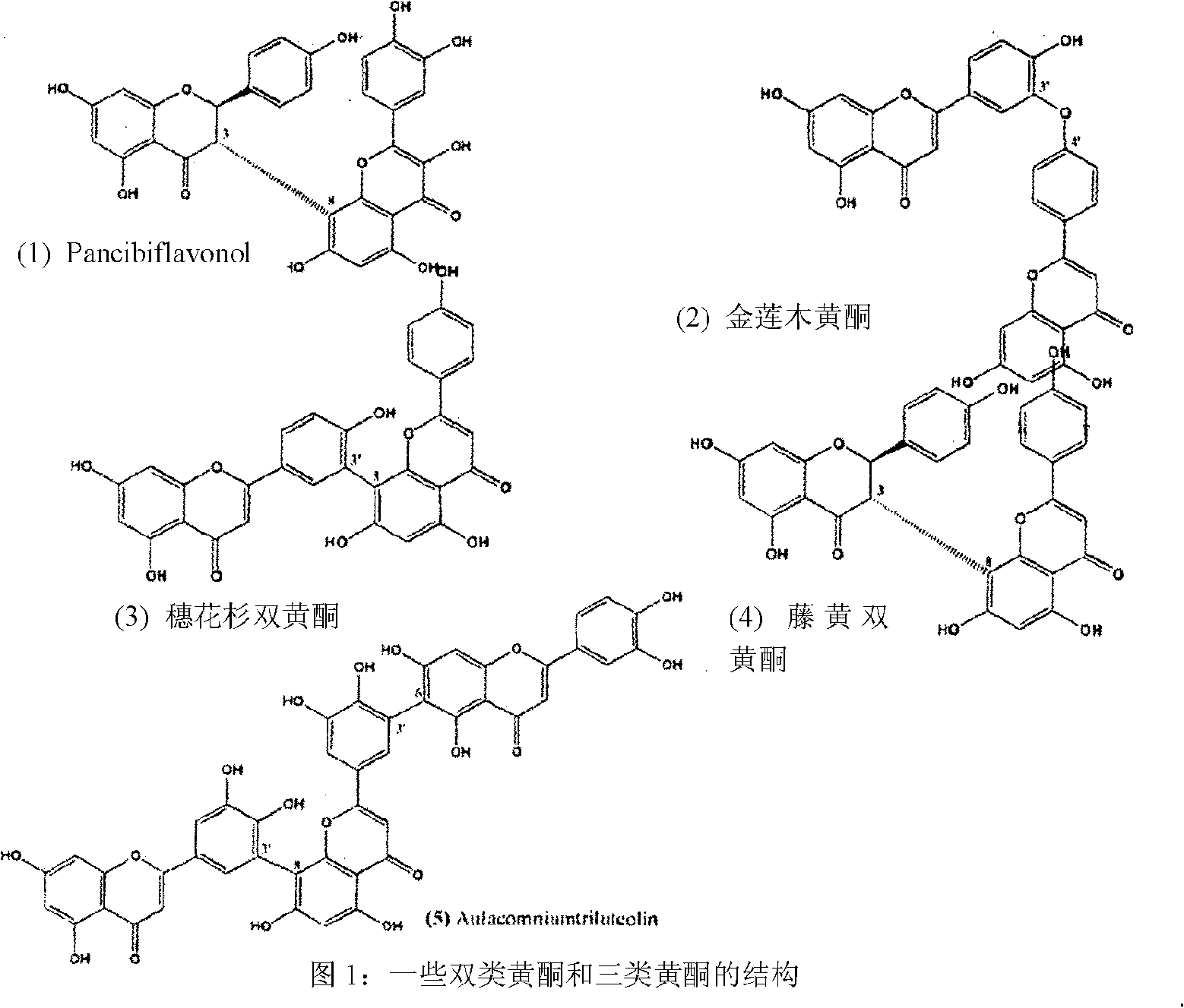
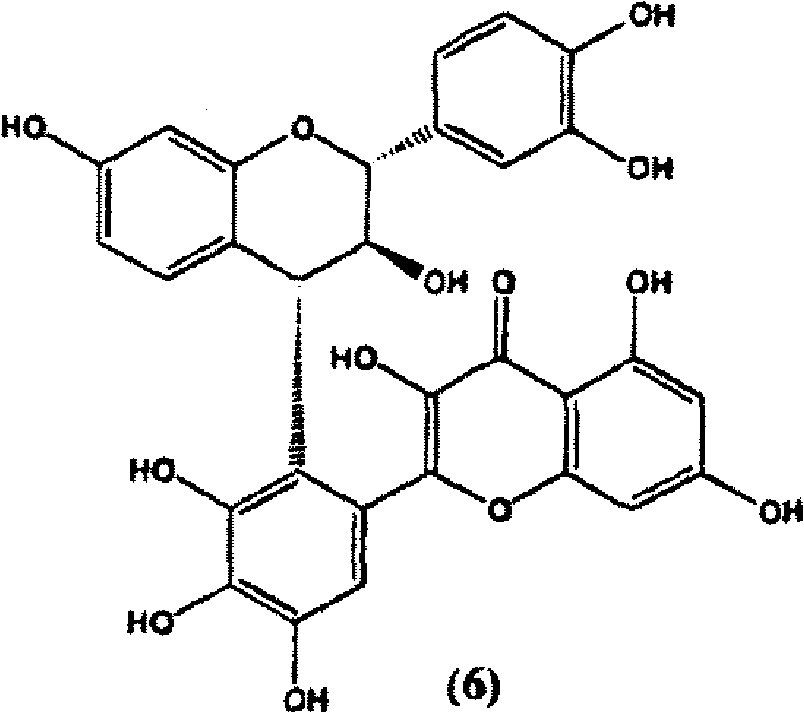
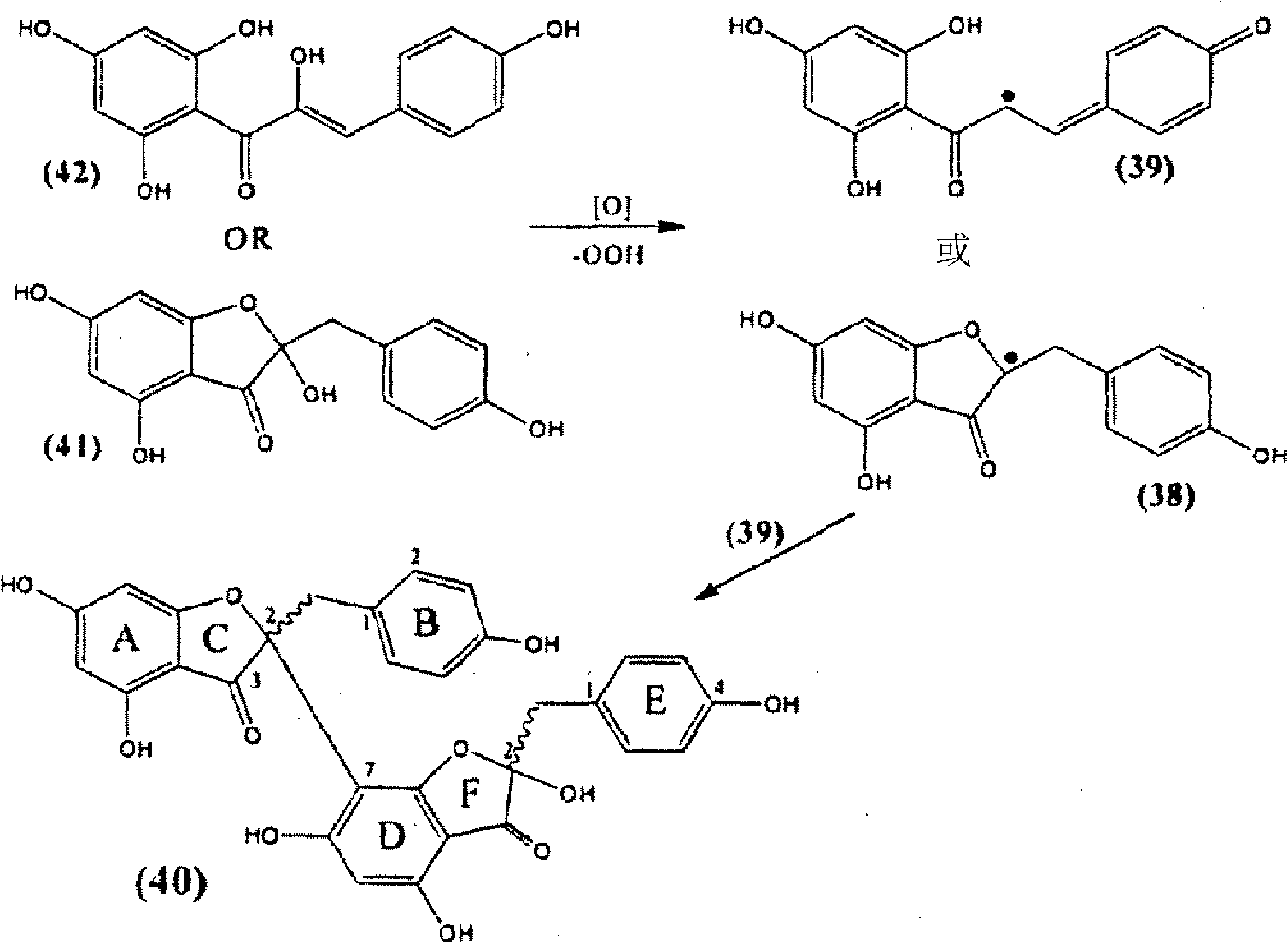
Synthesis of C-3 coupled biflavonoids and C-3 coupled biflavonoid analogues
A technology of C-3 and flavonoids is applied in the field of biflavonoids and C-3-coupled biflavonoid analogs, which can solve problems such as hindering the progress of drug development and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0210] Example 1 Preparation of 3-arylflav-3-enes
[0211]
[0212] Dry (2R)-5,7,3',4'-tetra(methyloxy)flavan-3-one[1] (100 mg, 0.29 mmol) and 1,3,5-tri-O-methanol Phloroglucinol (120 mg, 0.710 mmol, 2.5 equiv) was dissolved in anhydrous dichloromethane (10 mL), and the mixture was cooled in an ice / NaCl bath. Tin(IV) chloride (1M solution in dichloromethane, 0.5 mL, 0.5 mmol, 1.7 equiv) was added dropwise to this solution and the reaction mixture was stirred under argon in a melted ice / NaCl bath 24 hours (TLC monitoring consumption of starting material). The reaction mixture was filtered on a pad of basic alumina and washed with ethyl acetate. Purification on a silica preparative TLC plate gave 3-(1,3,5-O-methylphloroglucinol)-5,7,3',4'-tetramethoxyflavin-3-ene[ 2] (R f 0.26, 70.2 mg, 49%).
[0213] 1 H NMR: δ(DMSO) 3.64-3.80(s, 21H, 7x OCH 3 ), 5.83 (br, 1H, 4-H), 6.03 (br s, 1H, 2-H), 6.26 (s, 2H, 3″ / 5″-H), 6.33 (d, J=2.0Hz, 1H , 6-H), 6.69 (dd, J=0.6, 2.0Hz, 1H...
Embodiment 2
[0216] Example 2 Preparation of 3-arylflavan-4-ones
[0217]
[0218] 3-(1,3,5-O-methoxyphloroglucinol)-5,7,3',4'-tetramethoxyflav-3-ene (2) (50mg, 0.10mmol) was dissolved in THF (5mL), and added OsO 4 (3.1 mg, 0.012 mmol) and N-methylmorpholine-N-oxide (56.2 mg, 0.48 mmol). The reaction mixture was stirred at room temperature under argon for 1-2 days (TLC monitored consumption of starting material). Sodium metabisulfite solution (10% in water) was added and the reaction mixture was stirred for 30 minutes. Then extracted 3 times with dichloromethane, with NaHCO 3 (saturated aqueous solution), NaCl (saturated aqueous solution) and washed with MgSO 4 dry. Evaporation left a crude material which was purified on a preparative TLC plate to give 3-(1,3,5-O-methoxyphloroglucinol)-5,7,3',4'- Tetramethoxyflavan-4-one (3) (15 mg, 0.29 mmol, 29%).
[0219] 1 H NMR: δ(CDCl 3 )3.35 (s, 3H, 4″-OMe), 3.63 (s, 6H, 2” / 6”-OMe), 3.77 (s, 3H, OMe), 3.80)s, 3H, OMe), 3.81 (s, 3H, OM...
Embodiment 3
[0222] Example 3 (2R)-3-[(2R,3S)-5,7,3',4'-tetra(methyloxy)-3-hydroxyflavan]-5,7,3',4' -Four Preparation of Methoxyflavin-3-ene (6)
[0223]
[0224] Dry (2R)-5,7,3',4'-tetra(methyloxy)flavan-3-one (1) (200 mg, 0.581 mmol) and (2R,3S)-5,7,3 ',4'-Tetra(methyloxy)flavan-3-ol (5) (400 mg, 1.162 mmol, 2 equiv) was dissolved in anhydrous dichloromethane (10 mL), and the mixture was dissolved in ice / NaCl Cool in a bath. Tin(IV) chloride (1M solution in dichloromethane, 1 mL, 1 mmol, 1.7 equiv) was then added dropwise to this solution, and the reaction mixture was stirred under argon in a melted ice / NaCl bath for 24 hours (TLC monitoring of consumption of starting material). The reaction mixture was then filtered on basic alumina and washed with ethyl acetate. Purification on silica preparative TLC plates (hexane-ethyl acetate 4:6) gave (2R)-3-[(2R,3S)-5,7,3′,4′-tetrakis(methyloxy yl)-3-hydroxyflavan]-5,7,3',4'-tetramethoxyflavan-3-ene (6) (Rf0.20, 40.1mg, 0.060mmol, 10%)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com