Therapeutic compounds and compositions, and methods of use thereof
A compound and pharmaceutical technology, applied in the field of Janus kinase such as JAK1 inhibitor compound, can solve problems such as defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0476]
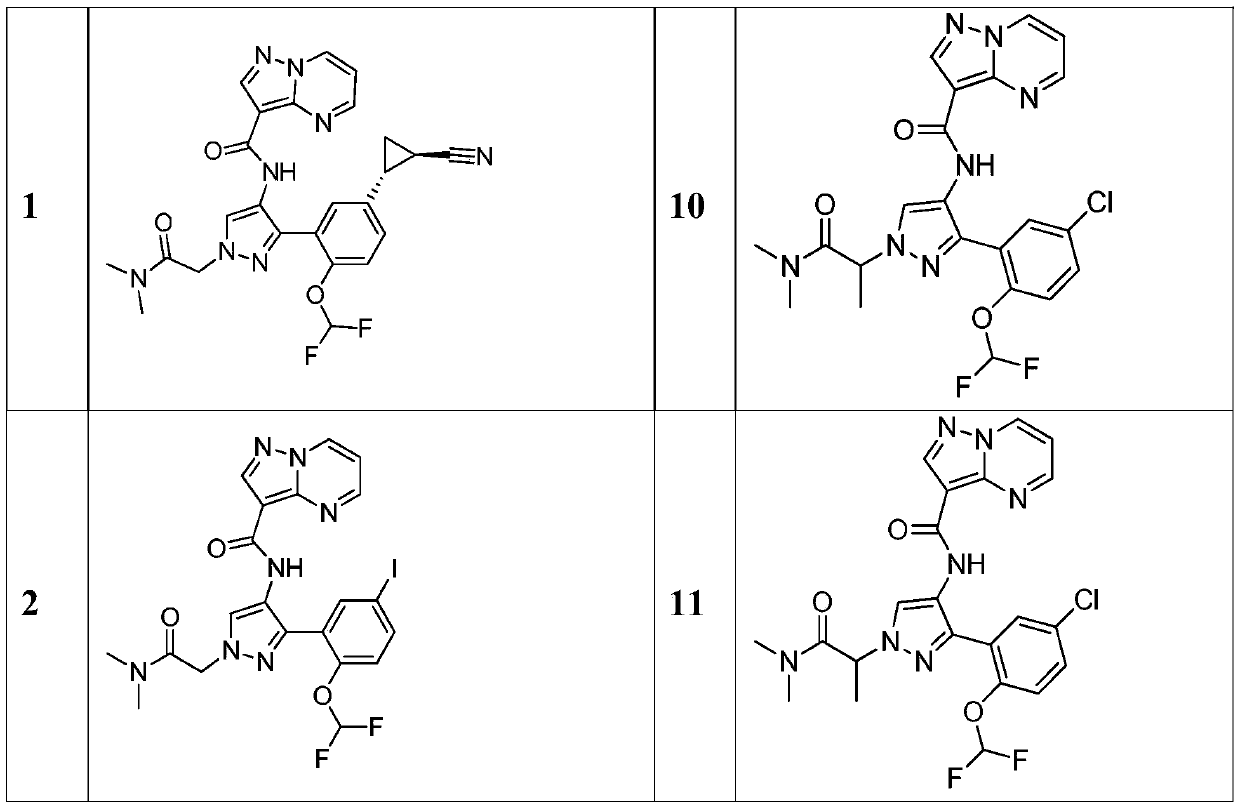
[0477] N-(3-(5-((1R,2R)-2-cyanocyclopropyl)-2-(difluoromethoxy)phenyl)-1-(2-(dimethylamino)-2 -Oxoethyl)-1H-pyrazol-4-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide
[0478] To N-(3-(5-((1R,2R)-2-cyanocyclopropyl)-2-(difluoromethoxy)phenyl)-1H-pyrazol-4-yl)pyrazolo To a solution of [1,5-a]pyrimidine-3-carboxamide (Intermediate 4, 100 mg, 0.230 mmol) in DMF (10 mL) was added Cs 2 CO 3 (160 mg, 0.491 mmol). Then 2-bromo-N,N-dimethylacetamide (80 mg, 0.482 mmol) was added. The resulting mixture was stirred in a 60°C oil bath for 30 minutes. The resulting mixture was concentrated under reduced pressure. The residue was purified by flash chromatography on silica gel eluting with dichloromethane / methanol (8% MeOH) to afford 20.9 mg (17%) of N-(3-(5-((1R,2R)-2-cyanocyclo Propyl)-2-(difluoromethoxy)phenyl)-1-(2-(dimethylamino)-2-oxoethyl)-1H-pyrazol-4-yl)pyrazolo [1,5-a]pyrimidine-3-carboxamide as a white solid. LC / MS (Method A, ESI): [M+H] + =521.3,R T = 1.50min; 1...
Embodiment 2
[0480]
[0481] N-[3-[2-(Difluoromethoxy)-5-iodophenyl]-1-[(dimethylcarbamoyl)methyl]-1H-pyrazol-4-yl]pyrazolo [1,5-a]pyrimidine-3-carboxamide
[0482] To N-[3-[2-(difluoromethoxy)-5-iodophenyl]-1H-pyrazol-4-yl]pyrazolo[1,5-a]pyrimidine-3-carboxamide ( To a solution of intermediate 3, 160 mg, 0.322 mmol) in DMF (4.0 mL) was added Cs 2 CO 3(210 mg, 0.645 mmol). To this mixture was added 2-bromo-N,N-dimethylacetamide (107 mg, 0.645 mmol). The resulting solution was stirred in a 60°C oil bath for 4 hours. The reaction mixture was partitioned between water and ethyl acetate. The aqueous phase was extracted with ethyl acetate (2x). The organic layers were combined, washed successively with water and brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was passed through a short pad of silica gel, eluting with dichloromethane / methanol (90 / 10). Appropriate fractions were combined and concentrated under reduced pressure. The crude...
Embodiment 3
[0484]
[0485] 2-amino-N-(3-(5-chloro-2-(difluoromethoxy)phenyl)-1-(2-(dimethylamino)-2-oxoethyl)-1H -pyrazol-4-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide
[0486] Step 1: N-[3-([3-[5-Chloro-2-(difluoromethoxy)phenyl]-1-[(dimethylcarbamoyl)methyl]-1H-pyrazole Synthesis of -4-yl]carbamoyl)pyrazolo[1,5-a]pyrimidin-2-yl]carbamate tert-butyl ester
[0487]
[0488] To N-[3-([3-[5-chloro-2-(difluoromethoxy)phenyl]-1H-pyrazol-4-yl]carbamoyl)pyrazolo[1,5- a] A solution of pyrimidin-2-yl] tert-butyl carbamate (200 mg, 0.385 mmol) in DMF (5.0 mL) was added with Cs 2 CO 3 (251 mg, 0.770 mmol) and 2-bromo-N,N-dimethylacetamide (63.0 mg, 0.379 mmol). The reaction mixture was stirred in a 60°C oil bath for 16 hours. The resulting mixture was concentrated under reduced pressure. The residue is purified by flash chromatography on silica gel, eluting with dichloromethane / methanol (95 / 5). The appropriate fractions were combined and concentrated under reduced pressure to give 200 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com