Tetrahydrofuran diquinoline compound, synthesis method and application thereof
A synthesis method and compound technology, applied in the field of medicine, can solve problems such as no report on synthesis, and achieve the effects of simple and easy-to-control synthesis method, significantly inhibiting activity, and short cycle.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018]
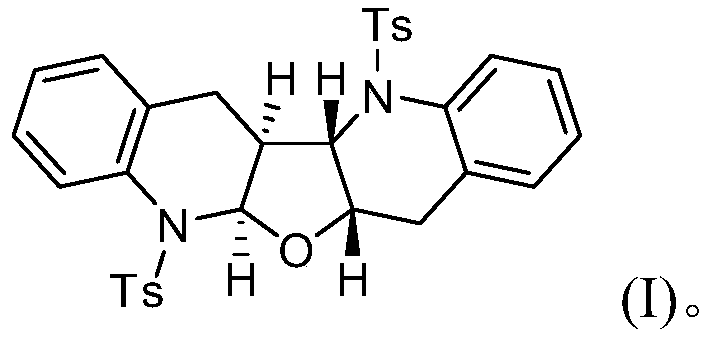
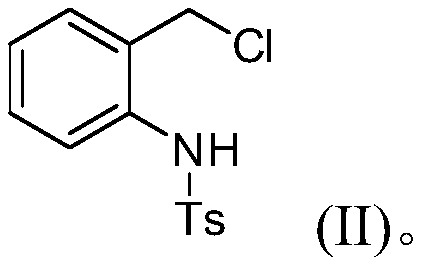
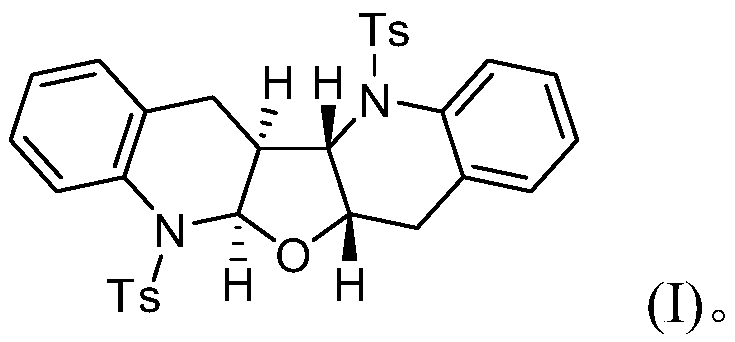
[0019] Concrete synthesis method: add N-(2-chloromethyl)phenyl-4-methylbenzenesulfonamide (1 is the compound shown in formula (II), 1mmol) and sodium carbonate (1mmol) in 10mL reaction tube, Then furan (2, 0.5mmol) and dichloromethane (2.0mL) were added, and it was stirred and reacted at 25°C for 6h; ), collect and combine the organic phases, filter after drying with anhydrous sodium sulfate, remove the solvent under reduced pressure, and separate the residue by silica gel column chromatography (petroleum ether / ethyl acetate=30:1~1:1, volume ratio), Compound 3 was obtained.
[0020] The obtained compound 3 is characterized as follows:
[0021] 1) White solid, 0.071g, 60% yield; mp: 232-233℃;
[0022] 2) 1 H NMR (400MHz, CDCl 3 ): δ7.62(d, J=7.6Hz, 1H), 7.57(d, J=8.0Hz, 1H), 7.42(d, J=8.0Hz, 2H), 7.30(d, J=8.0Hz, 3H ),7.20-7.14(m,6H),7.09(d,J=7.6Hz,2H),7.01(d,J=6.8Hz,1H),5.95(d,J=7.6Hz,1H),4.21(d ,J=7.6Hz,1H),4.10(d,J=7.6Hz,1H),2.77-2.72(m,2H),2.45(d,J=15.2Hz,...
Embodiment 2
[0029] Example 1 was repeated, except that sodium carbonate was replaced by tripotassium phosphate, dichloromethane was replaced by benzene, the reaction was carried out under ice-bath conditions, and the reaction time was changed to 36 hours. A white solid was finally obtained, 71% yield.
[0030] The resulting white solid was analyzed by H NMR, C NMR, IR, and high-resolution mass spectrometry, and was determined to be the target compound tetrahydrofuran [2,3-b][3',2'-b]biquinoline.
Embodiment 3
[0032] Repeat Example 1, the difference is: replace sodium carbonate with cesium hydroxide, replace dichloromethane with a composition of tetrahydrofuran and ethyl acetate (the volume ratio of tetrahydrofuran and ethyl acetate is 1:1), and change the reaction at 50°C conditions, the reaction time was changed to 10h. Finally a white solid was obtained, 51% yield.
[0033] The resulting white solid was analyzed by H NMR, C NMR, IR, and high-resolution mass spectrometry, and was determined to be the target compound tetrahydrofuran [2,3-b][3',2'-b]biquinoline.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap