Anti-MG7-Ag monoclonal antibody of and application thereof
A monoclonal antibody, mg7-ag technology, applied in the field of immunology, can solve problems affecting diagnosis and therapeutic applications, and achieve high affinity and antigen specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
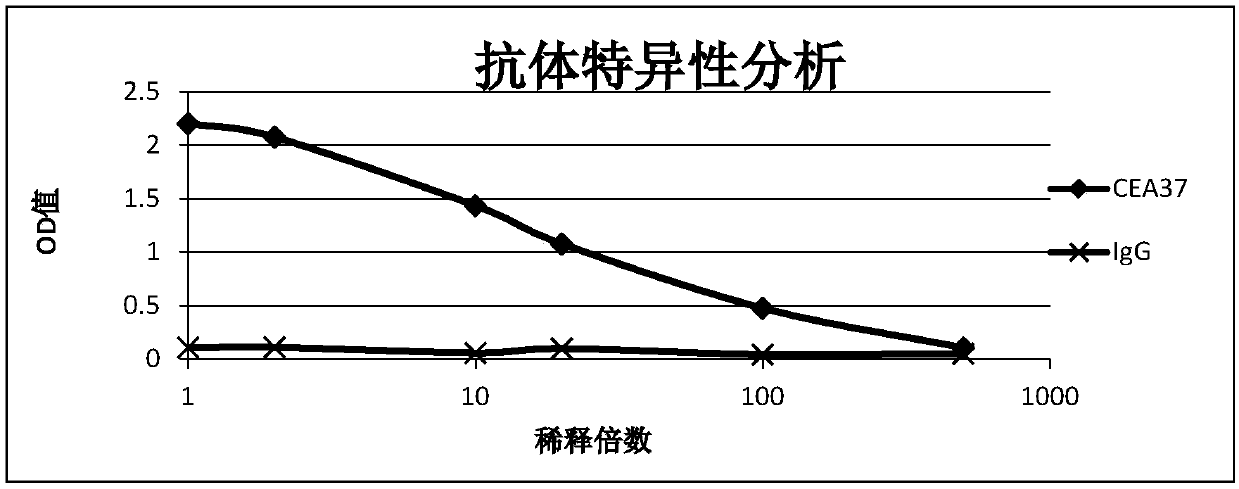
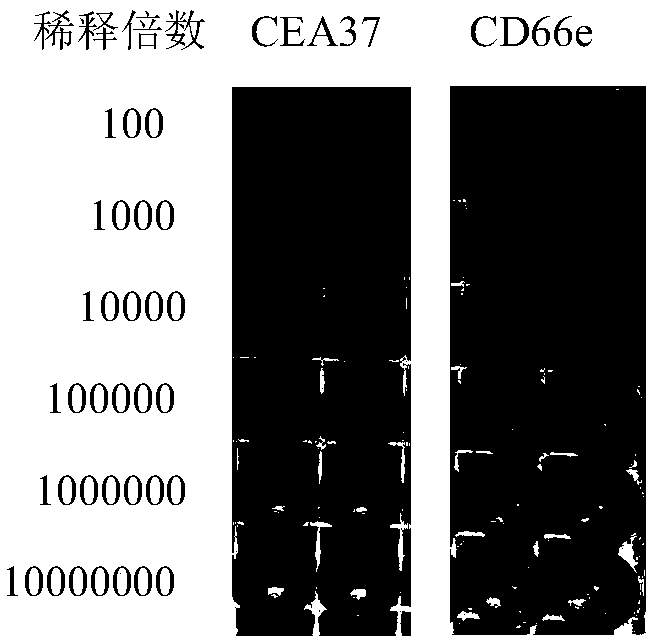
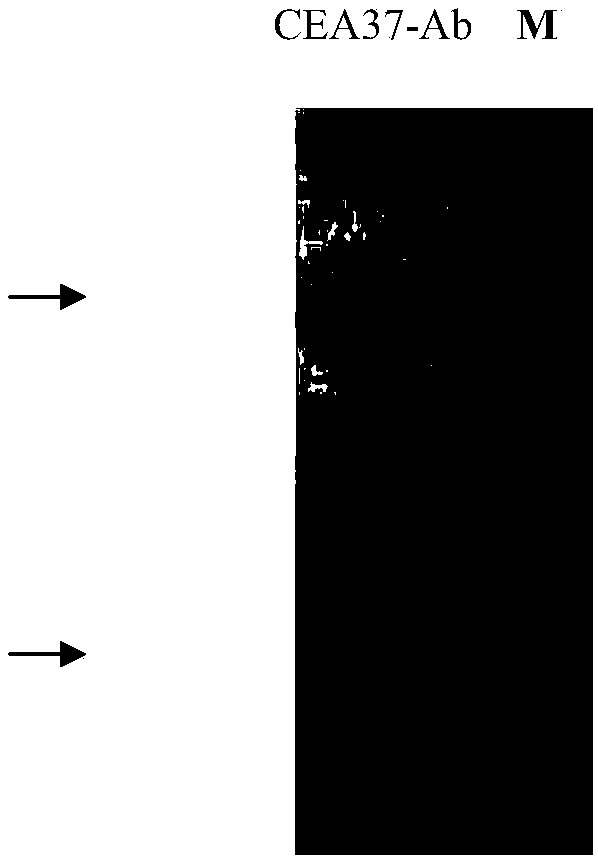
[0033] Example 1 Preparation and Identification of Anti-MG7-Ag Monoclonal Antibody CEA37-Ab
[0034] 1. Immunity and cell fusion:
[0035] Monoclonal antibody CEA37-Ab was obtained by immunizing Balb / c mice with eukaryotically expressed and purified CEACAM5 protein.
[0036] The specific method is as follows:
[0037] Three six-week-old Balb / c mice (purchased from Beijing Weitong Lihua) were immunized each time, and the second immunization was carried out at an interval of 4 weeks. The intraperitoneal immunization was boosted 3 days before the fusion, and the antibody titer of the mice was determined, and the antibody titer was selected. The splenocytes of the mouse with the highest valence were used for cell fusion: the fusion agent was 50% PEG4000, and the ratio of SP2 / 0 to splenocytes was 1:10 for fusion, and RPMI-1640 culture medium containing HAT was added, placed in a 96-well plate 5%CO 2 , and cultured in a 37°C constant temperature incubator.
[0038] Screening o...
Embodiment 2
[0044] Example 2 Preparation and Purification of Ascites of Anti-MG7-Ag Monoclonal Antibody
[0045] 1. Preparation of ascites: 10-week-old male BALB / c mice (purchased from Beijing Weitong Lihua) were intraperitoneally injected with 0.5 mL of liquid paraffin, and 10 days later, each mouse was intraperitoneally injected with 1X106 hybridoma cell suspension washed with normal saline. The solution was collected after ascites accumulated in mice, and stored at -20°C after aliquoting.
[0046] 2. Purification: Use ProteinG column (purchased from GenScript, L00209) for purification, collect 10 mL of each ascites, centrifuge at 3000 rpm for 10 min, absorb the supernatant, dilute 5 times with equilibrium buffer, filter with a 0.45 μm filter, and use a protein purifier (purchased from AKTAPurifier) for purification, after washing away impurity proteins with equilibration buffer (PBS), elute with acidic eluent (100mmol / LGlycin-HCl) at pH 2.6, and collect OD 280 For components >0.5,...
Embodiment 3
[0047] Example 3 Using CEA37-Ab Immunohistochemical Method to Detect the Expression of MG7-Ag in Tissue Chips
[0048] 1. Human gastric cancer tissue microarrays (surgical specimens confirmed by pathology at Xijing Hospital of Fourth Military Medical University) were selected. Dewax with xylene to gradient alcohol dehydration, wash with PBS twice, 5min each time;
[0049] 2. Freshly prepared 3% H 2 o 2 10min at room temperature; then wash 3 times with PBS, 5min each time;
[0050] 3. Block with 10% normal sheep serum for 30 minutes at room temperature;
[0051] 4. Carefully aspirate and discard the blocking solution without washing, add appropriate concentration of CEA37-Ab to the slices respectively, and incubate overnight at 4°C. The slices were then washed 3 times with PBS;
[0052] 5. Add HRP-labeled goat anti-mouse IgG working solution dropwise on the slice, and incubate at 37°C for 1 hour;
[0053] 6. Wash the slices twice with PBS, soak the slices in the substra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com