Hybridoma cell strain, monoclonal antibody generated by hybridoma cell strain and application
A hybridoma cell line and monoclonal antibody technology, applied in biochemical equipment and methods, microorganisms, biomaterial analysis, etc., can solve the problems of false positives, false negatives, inability to distinguish specific types of antibodies, etc. Efficient secretion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: the preparation of hybridoma cell line
[0050] (1) Prepare and purify subgroup A gp85 recombinant protein by conventional methods;
[0051] (2) Dilute the purified gp85 recombinant protein of subgroup A to 1.0 mg / mL with sterilized PBS, and immunize 6-week-old female Blab / C mice by intraperitoneal injection, 0.2 mL / mouse, four times.
[0052] Mix the above recombinant protein with complete / incomplete Freund's adjuvant in equal volume. The 3rd and 5th w booster immunization after the first immunization, the protein dose used was the same as the first immunization. The adjuvant used for the first immunization was complete Freund's adjuvant, and the adjuvant was changed to incomplete Freund's adjuvant for booster immunization. One week after the third immunization, blood was collected from the tail vein of the mice, the serum was separated, and the antibody level of the mice was detected. Four weeks after the third immunization, a booster immunization was...
Embodiment 2
[0077] Example 2: Preparation of ascites antibody using hybridoma cells
[0078] (1) Select healthy non-immunized 10-week-old female Balb / c mice, and inject liquid paraffin intraperitoneally 1-2 weeks before hybridoma cell inoculation, 0.5 mL / mouse.
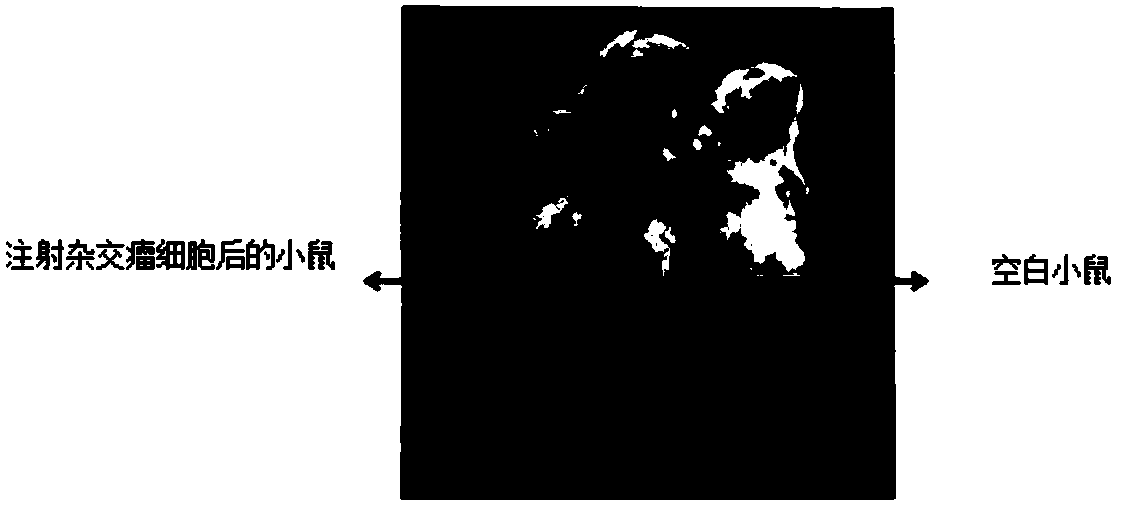
[0079] (2) Pipette the adherent hybridoma cells (preservation number: CGMCC NO.14290) into a single-cell suspension, wash the tumor cells with DMEM basal medium 2-3 times, and count and dilute the tumor cells after low-speed centrifugation up to 5×10 6 individual / mL. Slowly inject 0.2 mL of cell suspension intraperitoneally into the treated mice in multiple directions.
[0080] (3) After injecting hybridoma cells for 10 days, observe the production of ascites in mice (such as figure 2 shown). If the abdomen is obviously swollen and the skin feels tense when you touch it with your fingers, you can use a syringe with a 16-gauge needle to slowly draw out the ascites. Generally, it can be collected 2-3 times continuously, with ...
Embodiment 3
[0082] Example 3: Specific detection of monoclonal antibodies
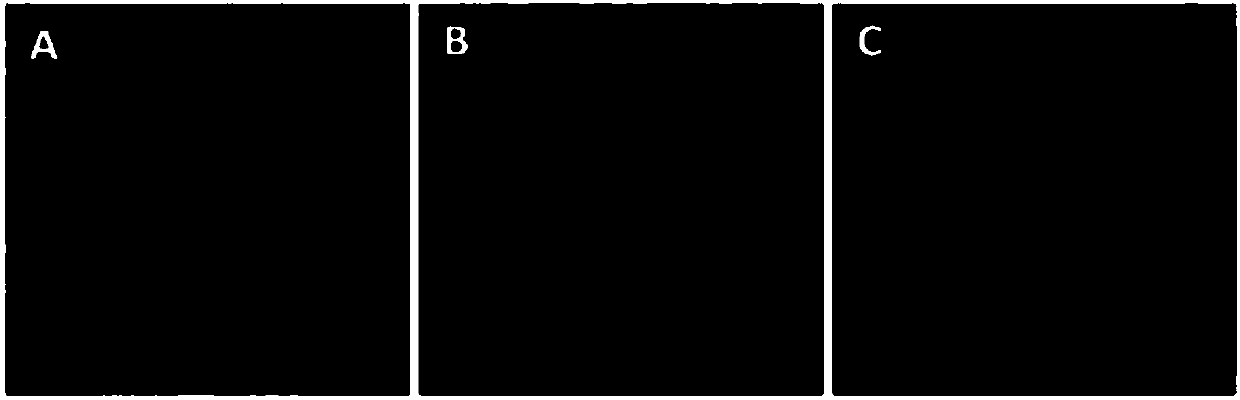
[0083] Using indirect immunofluorescence (IFA) to detect the specificity of the ascites antibody prepared in Example 2, the steps are as follows:
[0084] Chicken embryo fibroblasts (CEF) were grown in a 96-well cell culture plate. After the cells grew into a monolayer, the CEF cells were infected with ALV-A, ALV-B and ALV-J respectively. After 5-7 days of infection, they were treated with cold acetone -fixed with ethanol (6:4) for 5 minutes, washed once with PBS, dried in the air, and stored at -20°C as the antigen for detection. When performing IFA, properly dilute the ascites antibody to be detected and drop it on the virus-infected 96-well cell culture plate, incubate at 37°C for 30 minutes, wash 5 times with PBS, and then add goat anti-mouse IgG fluorescent labeling antibody, continue to incubate for 30 min, wash 5 times with PBS, and finally observe under a fluorescent microscope, the results are as follows...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com