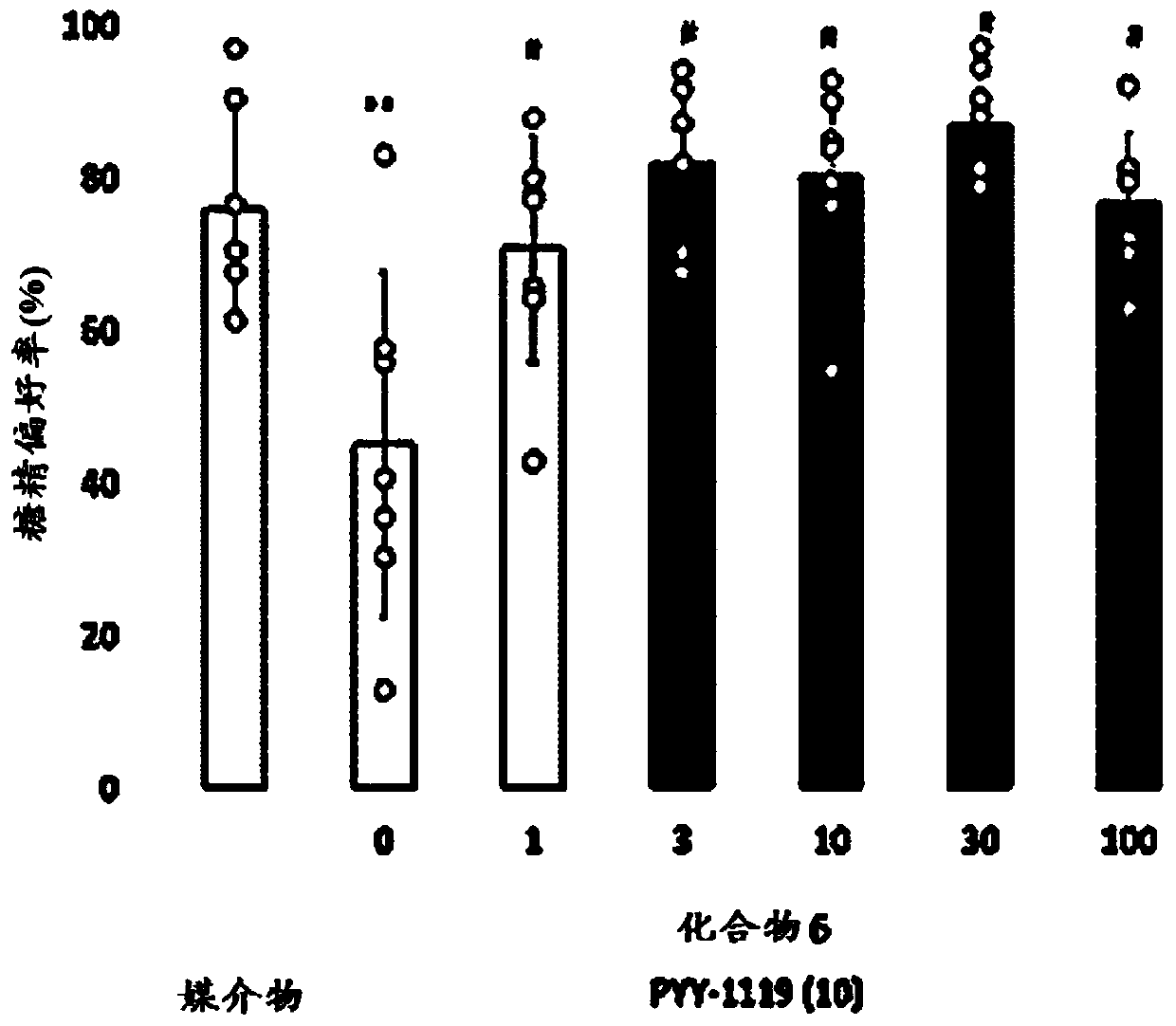
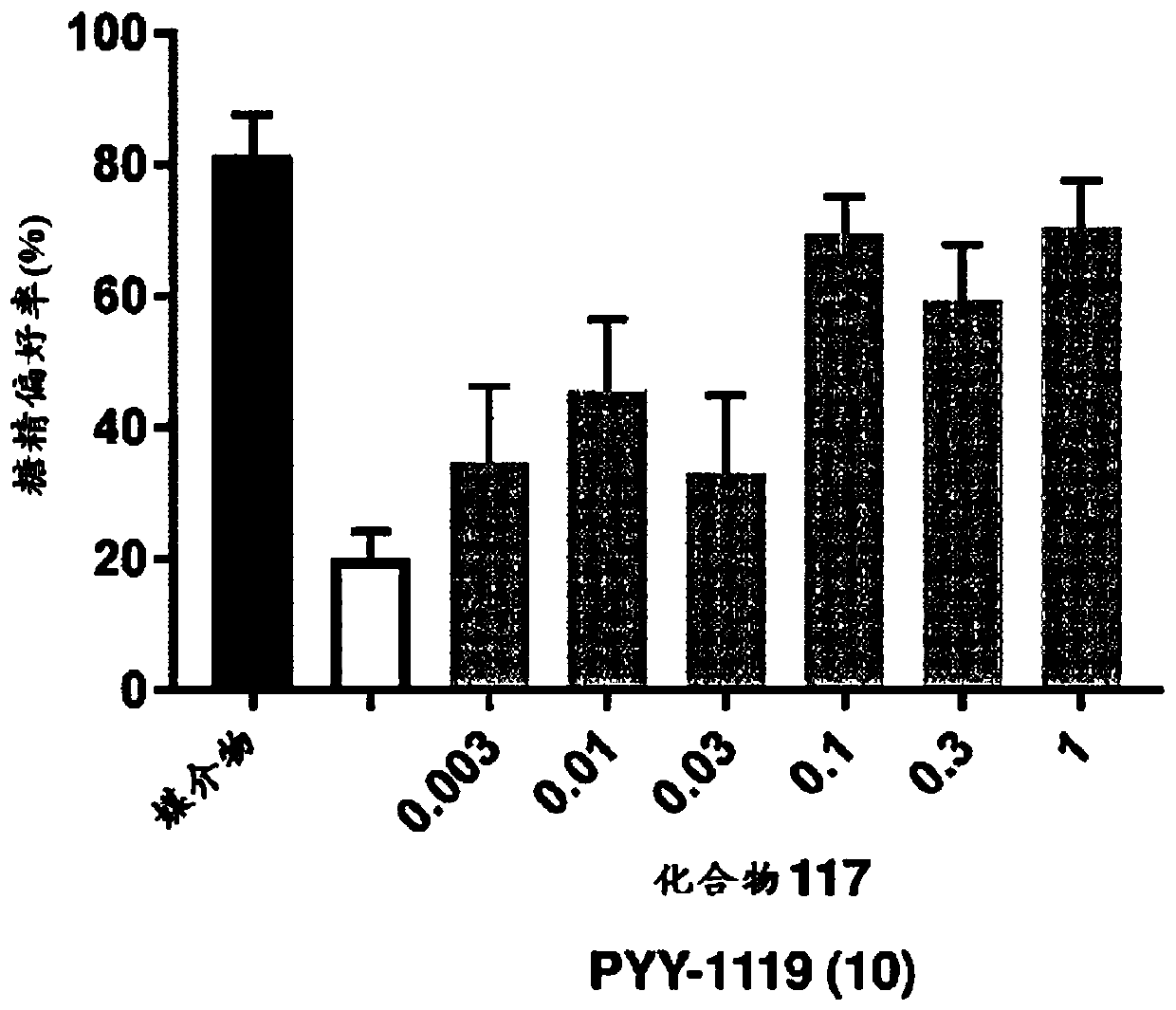
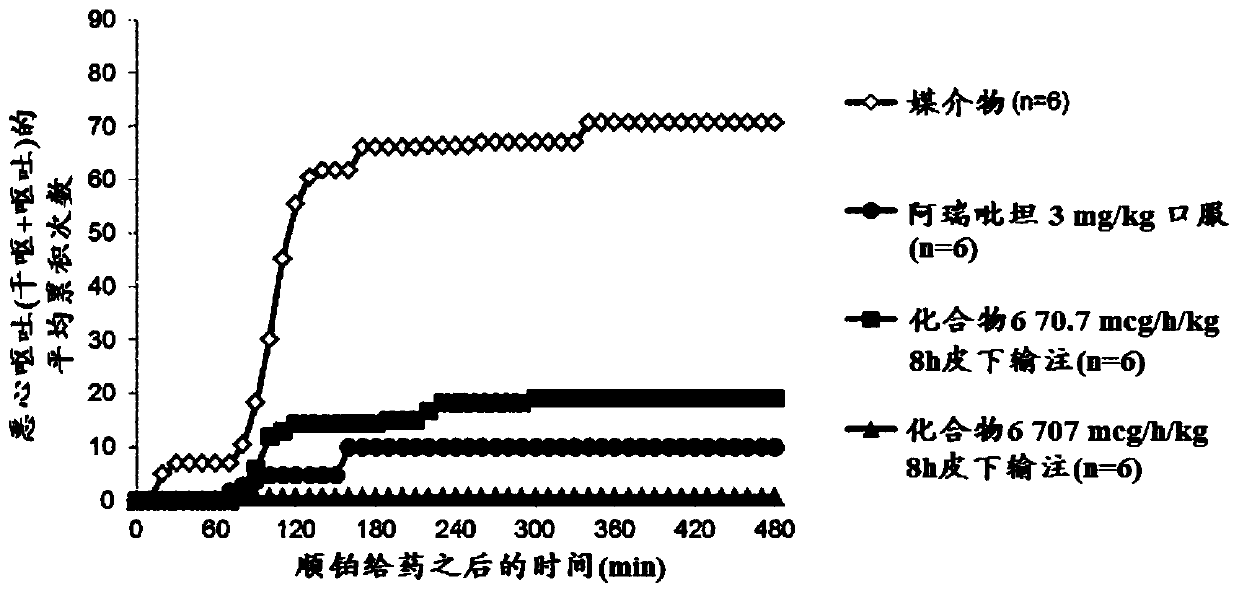
Gip receptor activating peptide
A representative, optional technique, applied in the field of novel peptide compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[1062] Compound (I) can be produced according to a peptide synthesis method known per se. The peptide synthesis method may be, for example, any of a solid-phase synthesis process and a liquid-phase synthesis process. That is, the target peptide can be produced by repeatedly condensing a partial peptide or amino acid capable of constituting the compound (I) and the remainder (which may be composed of two or more amino acids) according to a desired sequence. When the product with the desired sequence has a protecting group, the target peptide can be produced by eliminating the protecting group. Examples of known condensation methods and elimination methods of protecting groups include the methods described in (1) to (5) below.
[1063] (1) M. Bodanszky and M.A. Ondetti: Peptide Synthesis, Interscience Publishers, New York (1966)
[1064] (2) Schroeder and Luebke: The Peptide, Academic Press, New York (1965)
[1065] (3) Nobuo Izumiya et al.: Peptide Gosei-no-Kiso to Jikken (B...
Embodiment 1
[1269] Synthesis of H-Tyr-Aib-Glu-Gly-Thr-Val-Val-Ser-Leu-Tyr-Ser-Ile-Aib-Leu-Asp-Lys-Gln-Ala-Gln-Aib-Glu-Phe-Val-Lys -Trp-Leu-Leu-Lys-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-Lys-NH2 (SEQ ID NO: 12) (Compound 6)
[1270] Weigh the H-Glu(OtBu)-Phe-Val-Lys(Boc)-Trp(Boc)-Leu-Leu-Lys(Boc)-Gly-Gly-Pro-Ser(tBu)- prepared in Reference Example 1 Ser(tBu)-Gly-Ala-Pro-Pro-Pro-Ser(tBu)-Lys(Boc)-Sieber amide resin (0.220meq / g, 46mg) was put into a reaction tube and swelled with DMF. After removing DMF by filtration, Fmoc-Aib-OH (32.5 mg), 0.5M Oxyma in DMF (200 μL) and diisopropylcarbodiimide (15.9 μL) were successively added to the resin, and the mixture was shaken for 2 hours. The reaction solution was filtered off, and then the resin was washed 6 times with DMF. After negative confirmation in the ninhydrin test, a 20% piperidine solution in DMF was added thereto, and the mixture was shaken for 1 minute. The solution was filtered off, then 20% piperidine in DMF was added thereto ...
Embodiment 2
[1279] Synthesis of H-Tyr-Aib-Glu-Gly-Thr-Val-Val-Ser-Leu-Tyr-Ser-Ile-Aib-Leu-Asp-Lys-Gln-Ala-Gln-Aib-Glu-Phe-Val-Lys -Trp-Leu-Leu-Lys-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-Lys-NH 2 (acetate ester of compound 6)
[1280] The powder (1084.2 mg) prepared in the same manner as in Example 1 was dissolved in a small amount of acetonitrile-water, an ion exchange resin (AG1 X8 resin (acetate form), 1.2 meq / mL, 2.1 mL) was added thereto, and then The resulting mixture was allowed to stand for 1 hour with occasional shaking. After removing the resin by filtration, the filtrate was freeze-dried to obtain 929.6 mg of a white powder.
[1281] Mass spectrometry result: (M+H) + 4231.3 (calculated value 4231.3)
[1282] HPLC elution time: 7.5 minutes
[1283] Elution conditions:
[1284] Column Chromolith Performance RP-18e (100x 4.6mm ID)
[1285] Eluent: Use solution A: 0.1% TFA-water, and solution B: acetonitrile with 0.1% TFA, A / B: 95 / 5 to 35 / 65. Linear concentration gradie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com