PKM2 tetramer allosteric activation peptide and application thereof in reversing Warburg effect and chemosensitization in tumors
A tetramer, activating peptide technology, applied in the field of biomedicine, can solve the problem of not maintaining the persistence of PKM2 allosteric effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1P
[0039] Example 1 Preparation and molecular structure of PKM2 tetramer allosteric activation peptide
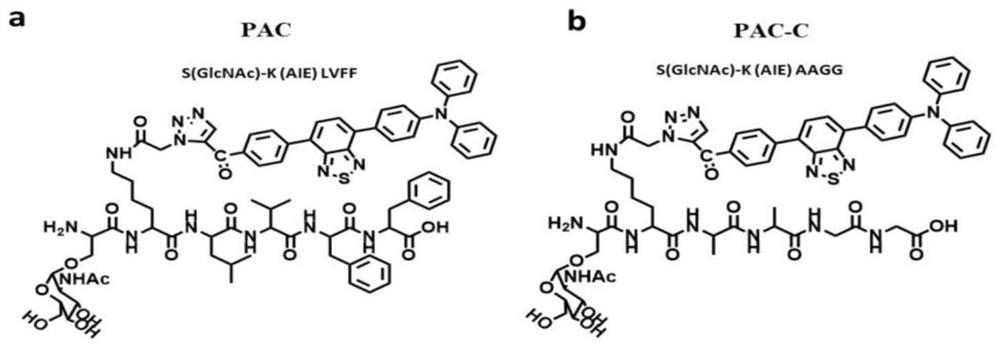
[0040] 1. Preparation of PKM2 tetramer allosteric activation peptide PAC ((S(GlcNAc)-K(TPA-1)LVFF), which can react with excess OGA enzyme in renal cell carcinoma (RCC) region to remove N-acetylglucosamine (GLcNAc) exposes serine, promotes the conversion of PKM2 from dimer to tetramer form, and self-assembles in situ to form water-insoluble nanofibers, achieve long-term retention and generate fluorescent signal, the PKM2 tetramer allosteric activation peptide It consists of the following three parts:
[0041] 1) The serine functional motif covered by N-acetylglucosamine (GLcNAc), the amino acid sequence of which is shown in formula I, can react with the OGA enzyme in the renal cell carcinoma (RCC) region to remove N-acetylglucosamine (GLcNAc) and then Expose serine to promote the conversion of PKM2 from dimer to tetramer;
[0042]
[0043] 2) The amino acid sequence that ...
Embodiment 2
[0048] Example 2 Polypeptide PAC undergoes allostery and self-assembly into water-insoluble nanofibers after removing N-acetylglucosamine (GLcNAc) through OGA enzyme reaction and exposing serine.
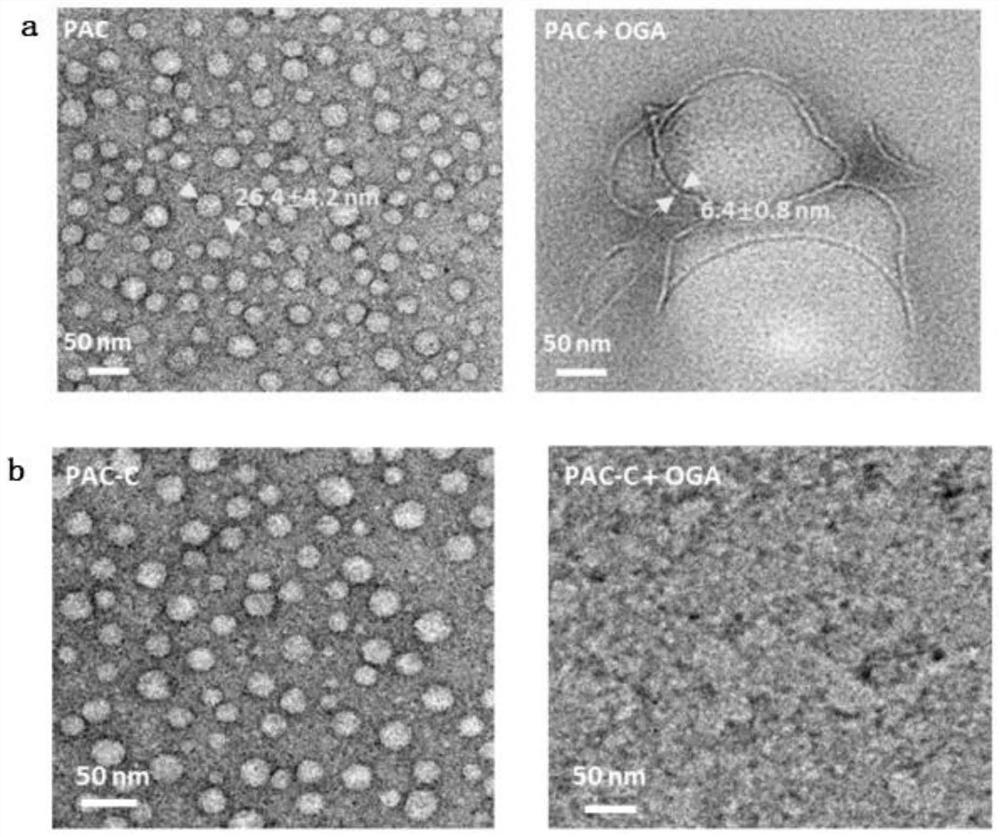
[0049] 1. Prepare a phosphate solution and gradually dissolve OGA to a pH of 6.5. Add PAC and PAC-C to the solution containing OGA enzyme, so that the final concentration of PAC and PAC-C is 100 μM. After 2 hours, use transmission electron microscopy to observe PAC and PAC-C solution samples.
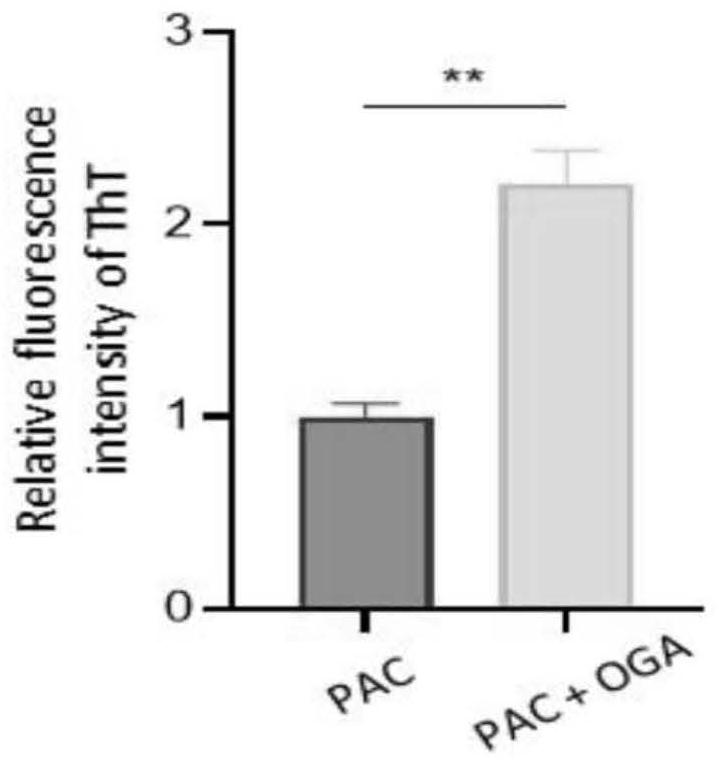
[0050] The result is as figure 2 From the results, it can be seen that the polypeptide PAC can transform and self-assemble into hydrophobic nanofibers in the solution containing OGA enzyme, while PAC-C cannot undergo allosteric and self-assembly behavior in the solution containing OGA enzyme.
[0051] 2. Prepare a phosphate solution and gradually dissolve OGA to a pH of 6.5. Add PAC to the solution containing OGA enzyme to make the final concentration of PAC 100 μM. After co-incubating for ...
Embodiment 3
[0053] Example 3 Cell test administration method
[0054] Humanized renal carcinoma cells 786-O and ACHN cells with high expression of OGA enzyme and normal renal cortical proximal convoluted tubule epithelial cells (HK-2 cells) were selected as control cells. The PAC and PAC-C polypeptides were dissolved in DMSO solvent to prepare a polypeptide nanomaterial solution with a solution concentration of 10 mM. The experimental cells in good condition and in logarithmic growth were randomly divided into PAC, PAC-C and PBS (phosphate buffered saline) groups. The effect of PAC-C and PBS solution on cell survival.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com