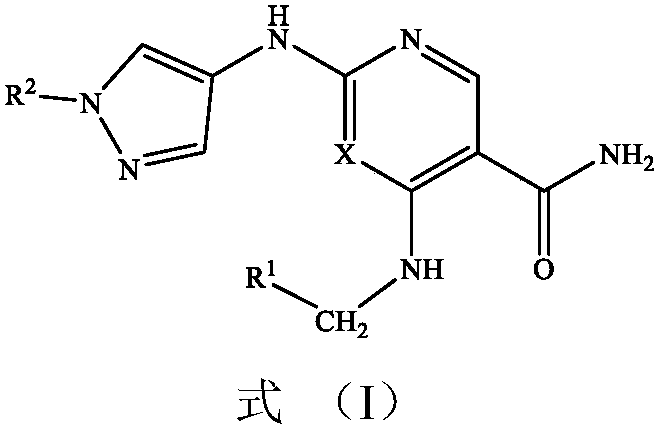
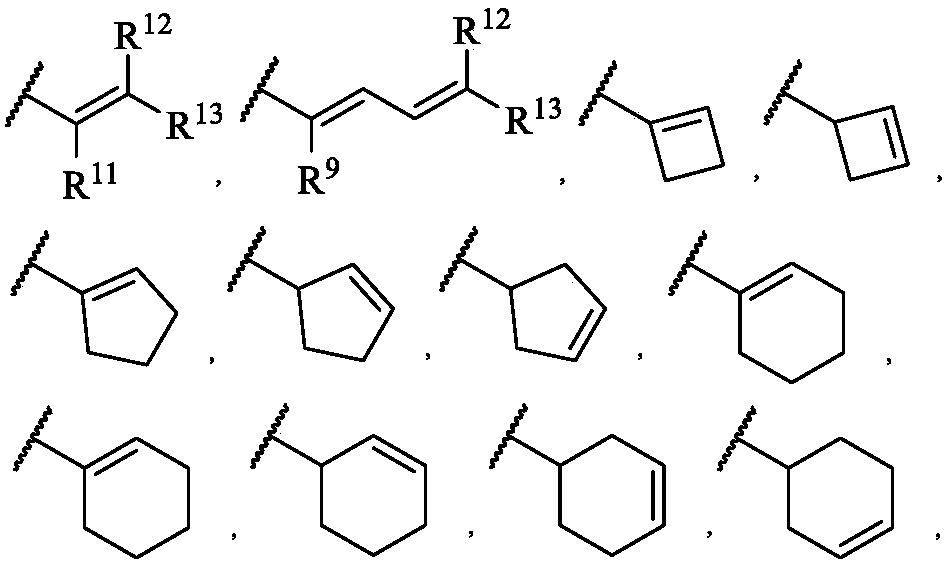
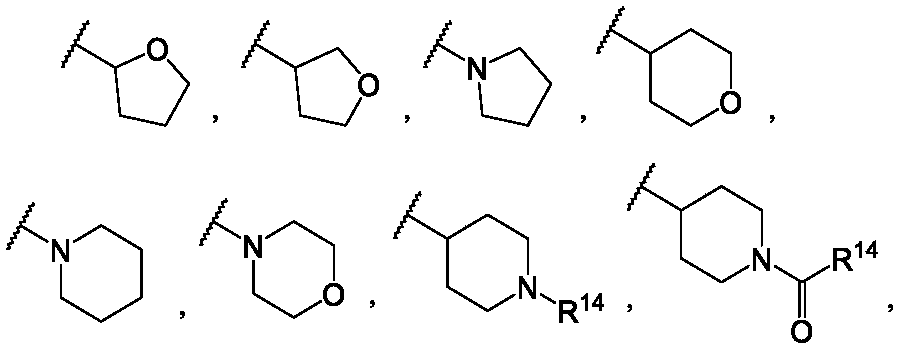
Vinyl-containing pyrimidine formamide compound, composition and application of compound and composition
A compound and formamide technology, applied in the field of medicinal chemistry, can solve problems such as high homology, low selectivity of JAK inhibitors, and small structural differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 Preparation of 4-(butyl-3-en-1-ylamino)-2-((1-(tert-butyl)-1H-pyrazol-4-yl)amino]pyrimidine-5-carboxamide :
[0071]
[0072] Step 1): Preparation of 2-chloro-4-(butyl-3-en-1-ylamino)pyrimidine-5-carboxamide:
[0073] Dissolve 2,4-dichloropyrimidine-5-carboxamide (400mg, 2.08mmol) and triethylamine (633mg, 6.25mmol) in tetrahydrofuran (10mL), add butyl-3-en-1-amine hydrochloride Salt (225mg, 2.1mmol), react at 25°C for 3 hours. Add saturated brine (200 mL), stir for 15 minutes, filter, and wash the filter cake with petroleum ether to obtain 380 mg of white solid, MS: 227 [M+H] + .
[0074]
[0075] Step 2): 4-(Butyl-3-en-1-ylamino)-2-((1-(tert-butyl)-1H-pyrazol-4-yl)amino]pyrimidine-5-carboxamide preparation:
[0076] Dissolve 2-chloro-4-(butyl-3-en-1-ylamino)pyrimidine-5-carboxamide (70 mg, 0.29 mmol) in sec-butanol (3 mL), add 1-tert-butyl-1H - Pyrazol-4-amine (49mg, 0.35mmol) and trifluoroacetic acid (0.1mL) were reacted at 100°C with locking tu...
Embodiment 2
[0077] Example 2 Preparation of 2-((1-tert-butyl-1H-pyrazol-4-yl)amino)-4-((2-methallyl)amino)pyrimidine-5-carboxamide:
[0078] The operation is the same as in Example 1, but butyl-3-en-1-amine hydrochloride in step 1) is replaced with 2-methylpropyl-2-en-1-amine to obtain a white solid, 1 H NMR (400MHz, DMSO-d 6 )δ10.36(s,1H),10.12(s,1H),8.55(s,1H),8.29–8.09(m,1H),7.98(s,1H),7.70–7.49(m,2H),4.90 –4.80(m, 2H), 4.10(d, J=5.7Hz, 2H), 1.76(s, 3H), 1.51(s, 9H). Chemical formula: C 16h 23 N 7 O, MS: 330 (M+H) + .
Embodiment 3
[0079] Example 3 2-((1-(tert-butyl)-1H-pyrazol-4-yl)amino)-4-((3-methylbutyl-2-en-1-yl)amino)pyrimidine- Preparation of 5-formamide:
[0080]
[0081] The operation is the same as in Example 1, and butyl-3-en-1-amine hydrochloride in step 1) is replaced with 3-methylbutyl-2-en-1-amine to obtain a white solid, 1 H NMR (400MHz, DMSO-d 6 )δ10.48(s,1H),9.94(s,1H),8.51(s,1H),8.36–8.10(m,1H),8.06(s,1H),7.81–7.40(m,2H),5.41 –5.22(m,1H),4.24–3.92(m,2H),1.71(s,3H),1.67(s,3H),1.50(s,9H).Chemical formula: C 17 h 25 N 7 O, MS: 344 (M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com