Urea substituted aromatic ring-dioxane quinazoline or quinoline type compound, composition and application of compound and composition
A technology of compounds and hydrates, which is applied in the fields of urea-substituted aromatic ring dioxaloquinazoline or quinoline compounds, compositions and their applications, and can solve problems such as failure to go on the market
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
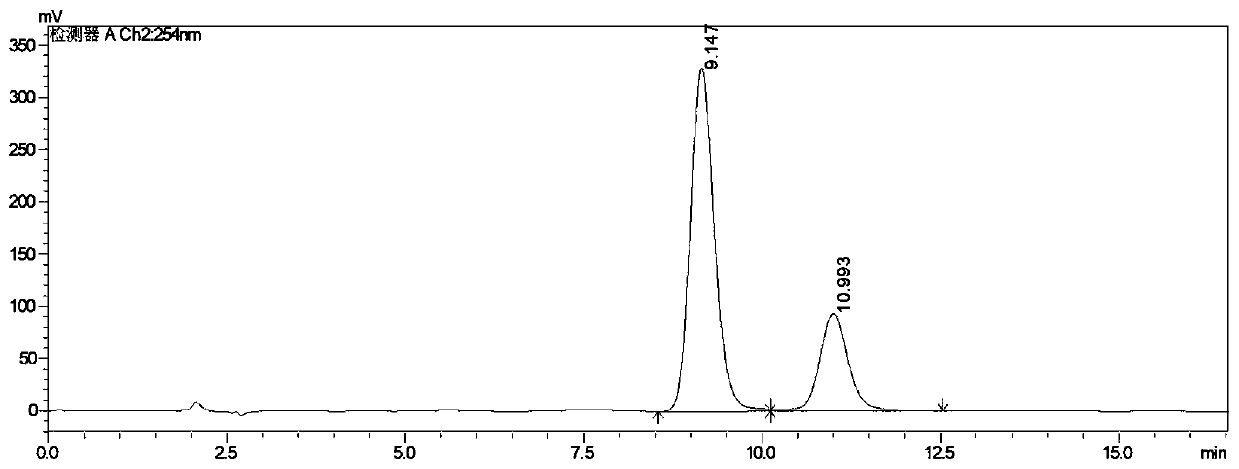
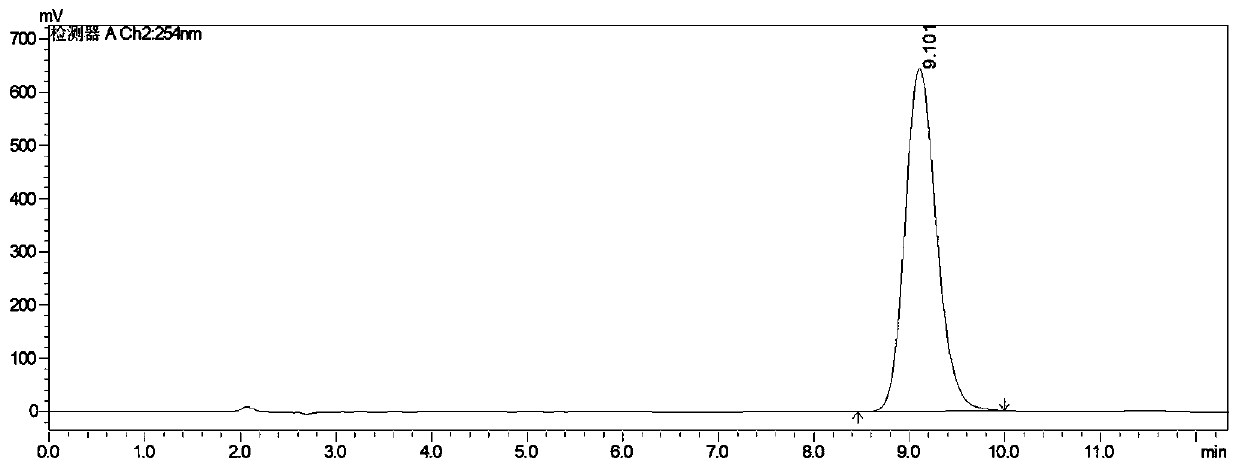
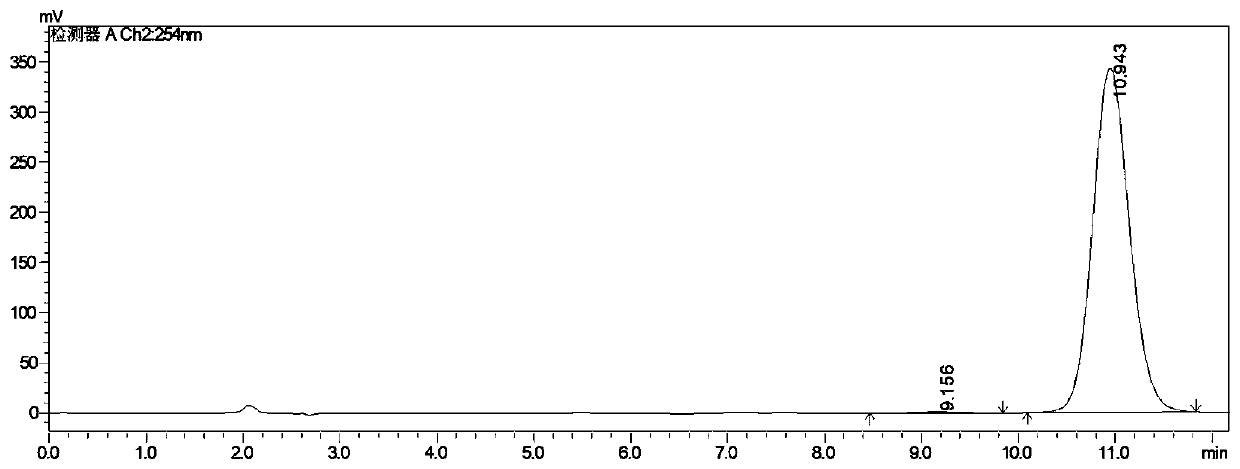
Image
Examples
preparation example Construction
[0095] Preparation of Intermediate 3.
[0096]
[0097] Step 1) A solution of 3-methoxyquinone (25.3g, 180mmol), potassium carbonate (104.5g, 756mmol), and 1,2-dibromoethane (74.4g, 396mmol) in DMF (100mL) Heated the reaction in a nitrogen system at 60°C for 6 hours. Quenched with water and extracted with ethyl acetate; the organic phase was washed with saturated sodium bicarbonate solution, dried over magnesium sulfate, filtered, and concentrated to give a dark gray oil: 5-methoxy -2,3-dihydrobenzo[b][1,4]dioxane (25.4g, 153mmol, yield 85%);
[0098] Step 2) under the ice-water bath condition of nitrogen atmosphere, to containing AlCl 3 (12.0g, 90mmol) in nitromethane (200mL) was slowly added dropwise acetyl chloride (5.57mL, 78mmol). Then slowly added dropwise 5-methoxy-2,3-dihydrobenzo[b][1, 4] Nitromethane (100mL) solution of dioxane (10.0g, 60mmol). The reaction was stirred at room temperature for 5 hours, and quenched by adding 1N hydrogen chloride solution. The org...
Embodiment 1
[0104] Example 1. 1-(1-(4-fluorophenyl)ethyl)-3-(4-((5-(3-morpholinopropyl)-2,3-dihydro-[1,4] Preparation of dioxane[2,3-f]quinazolin-10-yl)oxy)phenyl)urea
[0105]
[0106] Step 1): Under ice-water bath conditions, add phenyl chloroformate and pyridine respectively to the DMF solution of 1-(4-fluorophenyl)ethyl-1-amine, stir at room temperature for 8 hours, the product (1-(4 -Fluorophenyl) ethyl) phenyl carbamate was directly used in the next step, MS: 260 [M+H] +
[0107] Step 2): Add 4-aminophenol to the reaction solution obtained in step 1), heat the reaction at 50°C for two hours, cool, add water to quench, extract with ethyl acetate, wash with saturated brine, dry the organic phase, and concentrate to obtain a gray color The solid product 1-(1-(4-fluorophenyl)ethyl)-3-(4-hydroxyphenyl)urea was used directly in the next step;
[0108] Step 3): The product obtained in step 2), 10-chloro-5-(3-morpholine propoxy)-2,3-dihydro-[1,4]dioxane[2,3-f ] quinazoline (intermedi...
Embodiment 2
[0110] Example 2. 1-(1-(4-fluorophenyl)ethyl)-3-(2-methyl-4-((5-(3-morpholinopropyl)-2,3-dihydro- [1,4]dioxane[2,3-f]quinazolin-10-yl)oxy)phenyl)urea
[0111]
[0112] Operated with Example 1, the 4-aminophenol in step 2) was replaced by 4-amino-3-methylphenol to react to obtain a white solid product; 1 H NMR (300MHz, DMSO-d 6 )δ8.40(s,1H),7.84(d,J=6.0Hz,1H),7.70(s,1H),7.42-7.37(m,2H),7.18(t,J=9.0Hz,2H), 7.07(d,J=6.0Hz,1H),7.01-6.99(m,2H),6.92(d,J=9.0Hz,1H),4.85-4.80(m,1H),4.43-4.38(m,4H) ,4.20(br,2H),3.58(br,4H),2.45-2.38(m,6H),2.19(s,3H),1.96(br,2H),1.39(d,J=6.0Hz,3H). MS:618[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com