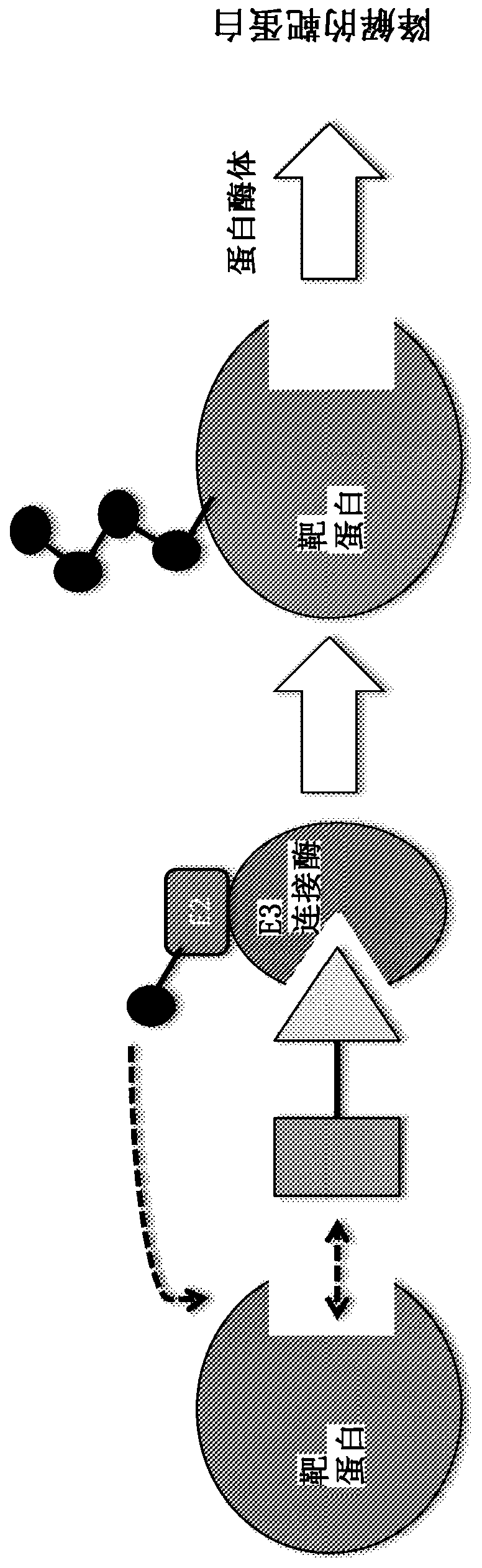
Compounds and methods for the targeted degradation of rapidly accelerated fibrosarcoma polypeptides
A technology of bifunctional compounds and solvates, applied in the field of compounds and methods for rapidly accelerating the targeted degradation of fibrosarcoma polypeptides, can solve problems such as inability to target BRaf inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
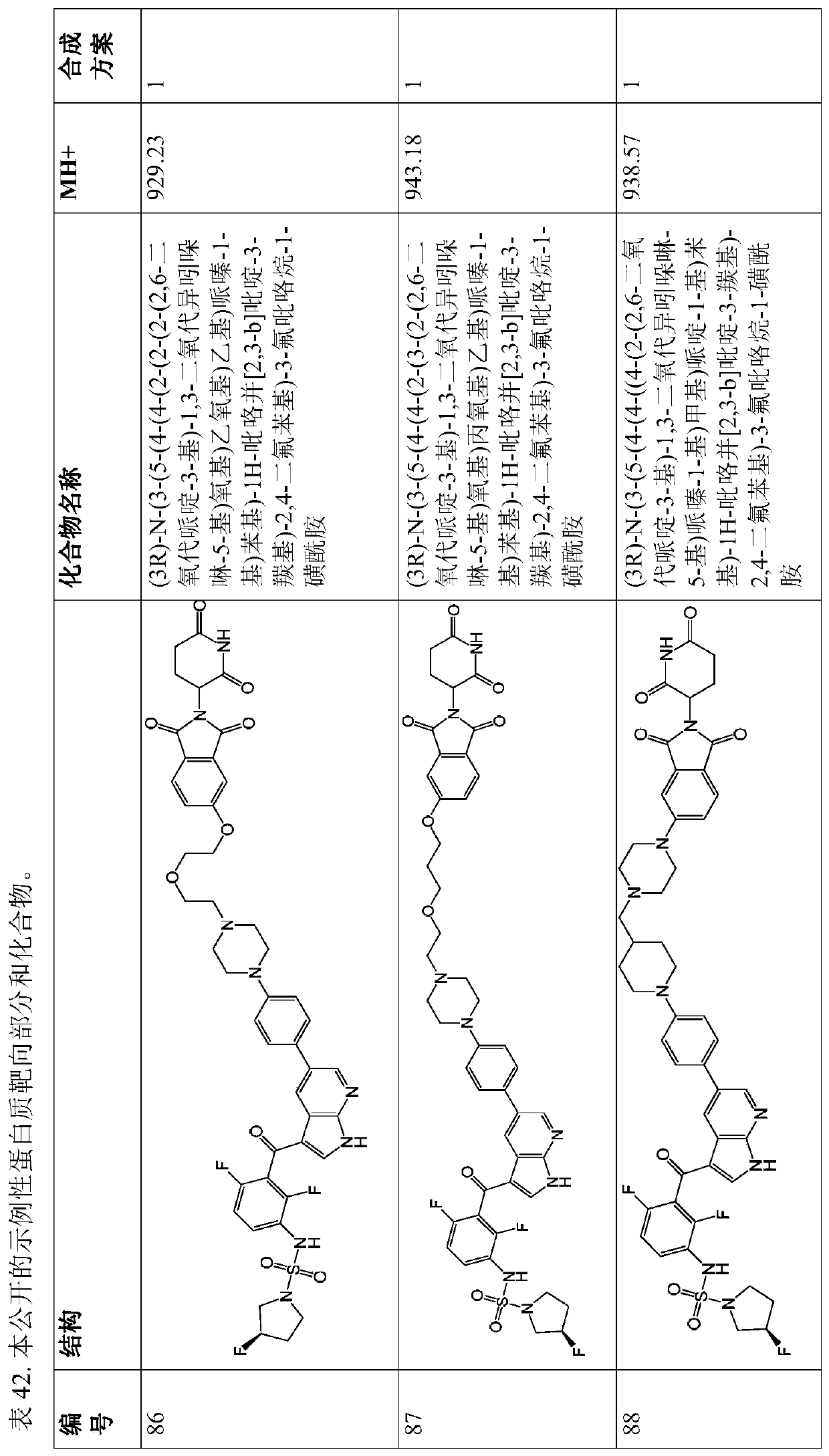
[2446] Assay and Degradation Data
[2447] Protocol for cellular assay of target protein degradation (A375 cells). A375 cells were cultured in ATCCDMEM+10%FBS in 12-well plates, and treated with the compounds shown in Tables 1-41 or 0.1% DMSO vehicle control for 16 hours. Cells were harvested in Cell Signaling Lysis Buffer (Cat. No. 9803) supplemented with Roche Protease Inhibitor Tablets (Cat. No. 11873580001), and the lysate was clarified by microcentrifugation. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes using the Invitrogen iBlot system. Immunoblots were performed for BRAF (Santa Cruz Cat. No. 9002), CRAF (BD Cat. No. 610151 ) and pErk (Cell Signaling Cat. No. 9106). GAPDH (catalog no.) was used as a loading control. Quantification was performed using BioRad Image Lab 5 software.
[2448] Protocol for In-Cell Western cellular assay of target protein degradation (A375 cells). A375 cells were cultured in ATCC DMEM+10% FBS in 96-well p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com