Preparation method for 2-4-6-trimethylbenzoyl chloride
A technology of trimethylbenzoyl chloride and trimethylbenzoic acid, applied in the field of preparation of 2-4-6-trimethylbenzoyl chloride, can solve the problems of slow reaction, environmental pollution, insufficient recycling of by-products and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Put 1000kg of thionyl chloride into the acid chloride synthesis kettle, start the hydrogen chloride and sulfur dioxide absorption system, and prepare hydrochloric acid and sodium sulfite. Control the temperature of the acid chloride synthesis kettle at 25°C, slowly add 500kg of 2-4-6-trimethylbenzoic acid into the acid chloride synthesis kettle, after the feeding is completed, the acid chloride synthesis kettle is heated to a reflux state, kept at 50°C for 2 hours, and kept At the end, switch the reflux state of the acid chloride synthesis tank to the recovery of thionyl chloride, and the recovered thionyl chloride is used for the synthesis of the next batch of acid chlorides. The recovery temperature is controlled at 60°C. In the rectifying tower, the acid chloride is distilled under negative pressure to obtain the finished product of 2-4-6-trimethylbenzoyl chloride with a purity of 98.9%.
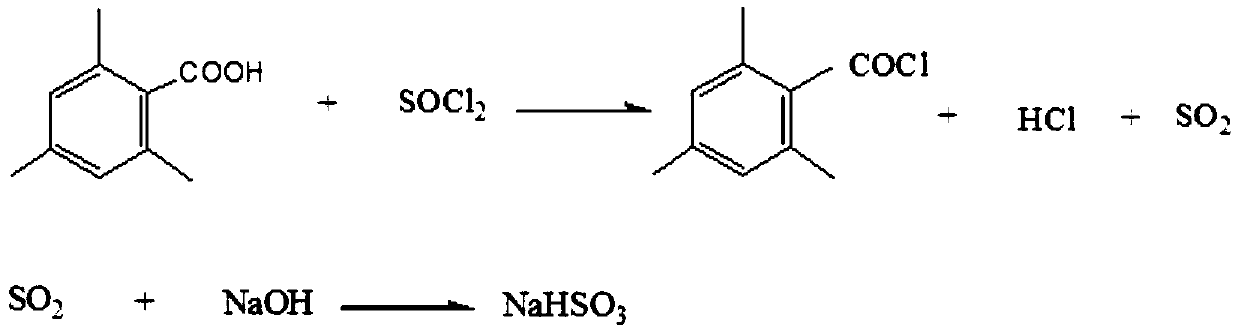
[0016] The reaction formula is as follows:
[0017]
Embodiment 2
[0019] Put 1500kg of thionyl chloride into the acid chloride synthesis kettle, start the hydrogen chloride and sulfur dioxide absorption system, and prepare hydrochloric acid and sodium sulfite. Control the temperature of the acid chloride synthesis kettle at 50°C, slowly add 1000kg of 2-4-6-trimethylbenzoic acid into the acid chloride synthesis kettle, after the feeding is completed, the acid chloride synthesis kettle is heated to a reflux state, kept at 80°C for 4 hours, and kept At the end, switch the reflux state of the acid chloride synthesis tank to the recovery of thionyl chloride, and the recovered thionyl chloride is used for the synthesis of the next batch of acid chlorides. The recovery temperature is controlled at 80°C. In the rectifying tower, the acid chloride is evaporated under negative pressure to obtain the finished product of 2-4-6-trimethylbenzoyl chloride with a purity of 98.7%.
Embodiment 3
[0021] Preparation of 2-4-6-trimethylbenzoyl chloride
[0022] Put 2000kg of thionyl chloride into the acid chloride synthesis kettle, start the hydrogen chloride and sulfur dioxide absorption system, and prepare hydrochloric acid and sodium sulfite. Control the temperature of the acid chloride synthesis kettle at 80°C, slowly add 1500kg of 2-4-6-trimethylbenzoic acid into the acid chloride synthesis kettle, after the feeding is completed, the temperature of the acid chloride synthesis kettle is raised to a reflux state, and the temperature is kept at 100°C for 6 hours, and the heat preservation End, switch the reflux state of the acid chloride synthesis tank to the recovery of thionyl chloride, the recovered thionyl chloride is used for the next batch of acid chloride synthesis, the recovery temperature is controlled at 100 °C, and the recovery is completed, the crude acid chloride in the acid chloride synthesis tank is transferred to the acid chloride In the rectifying tower...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com