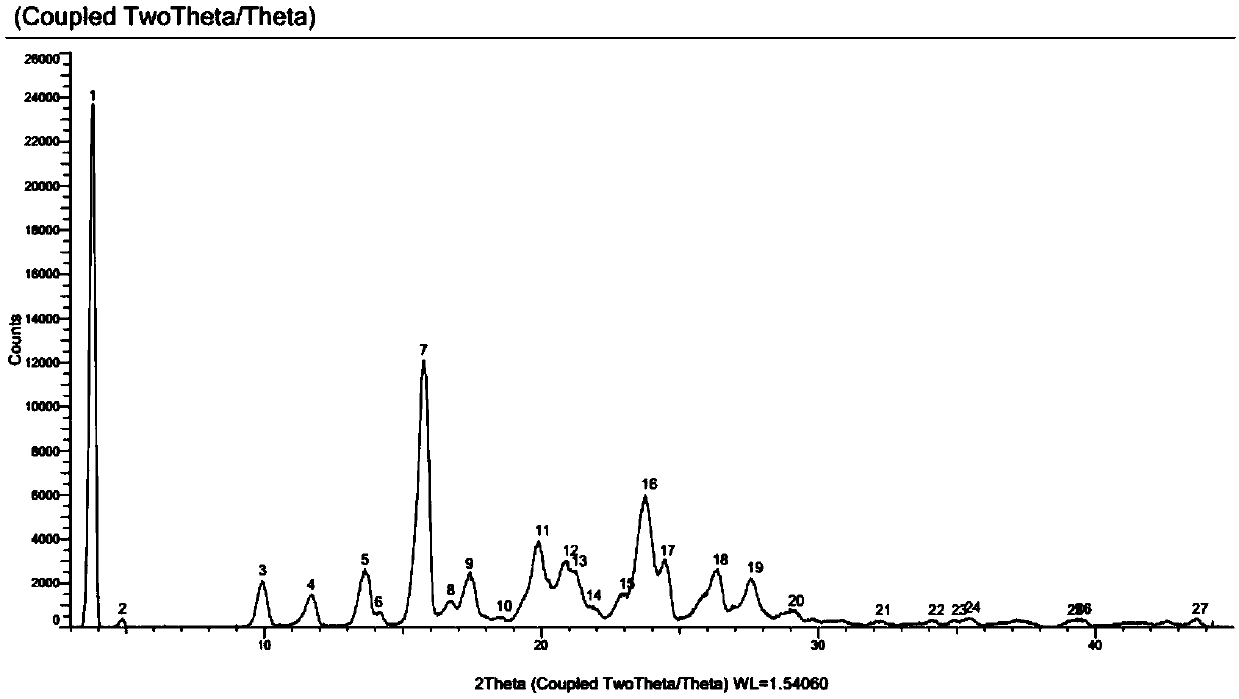
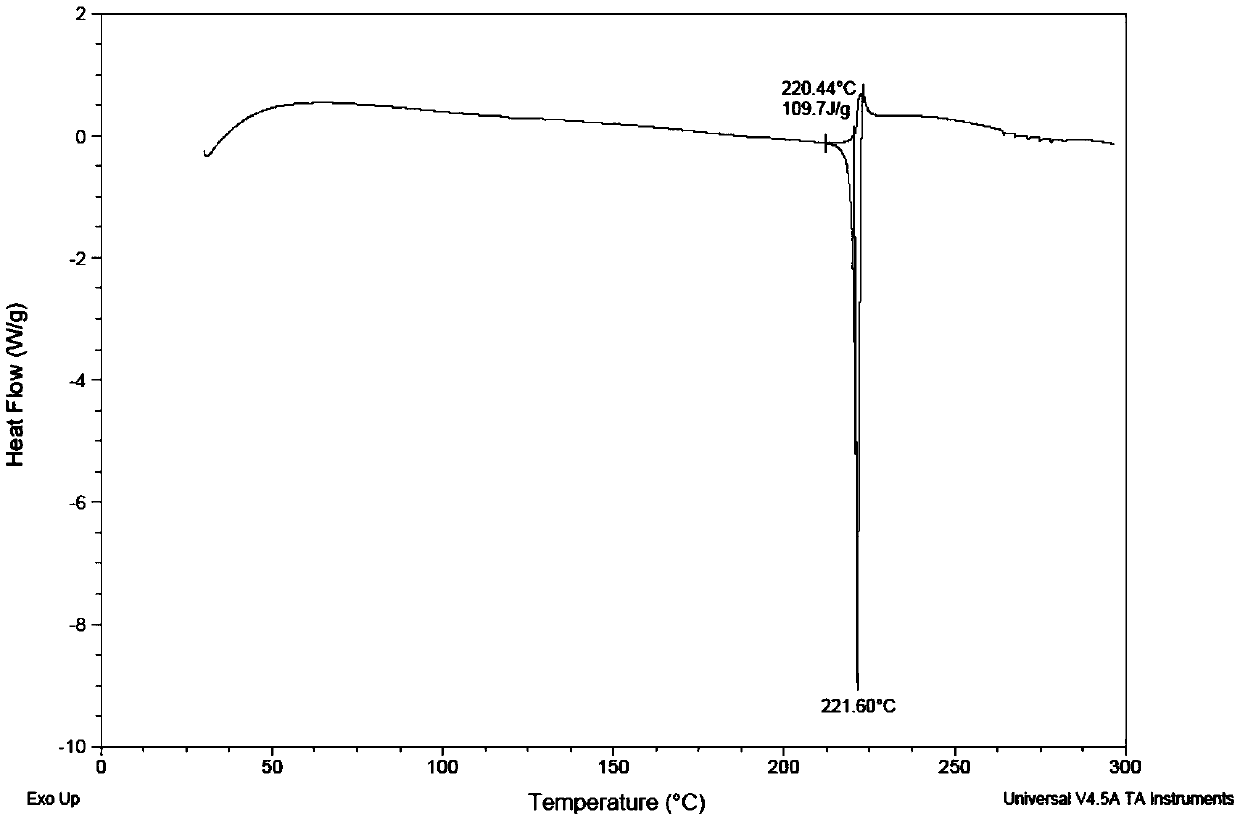
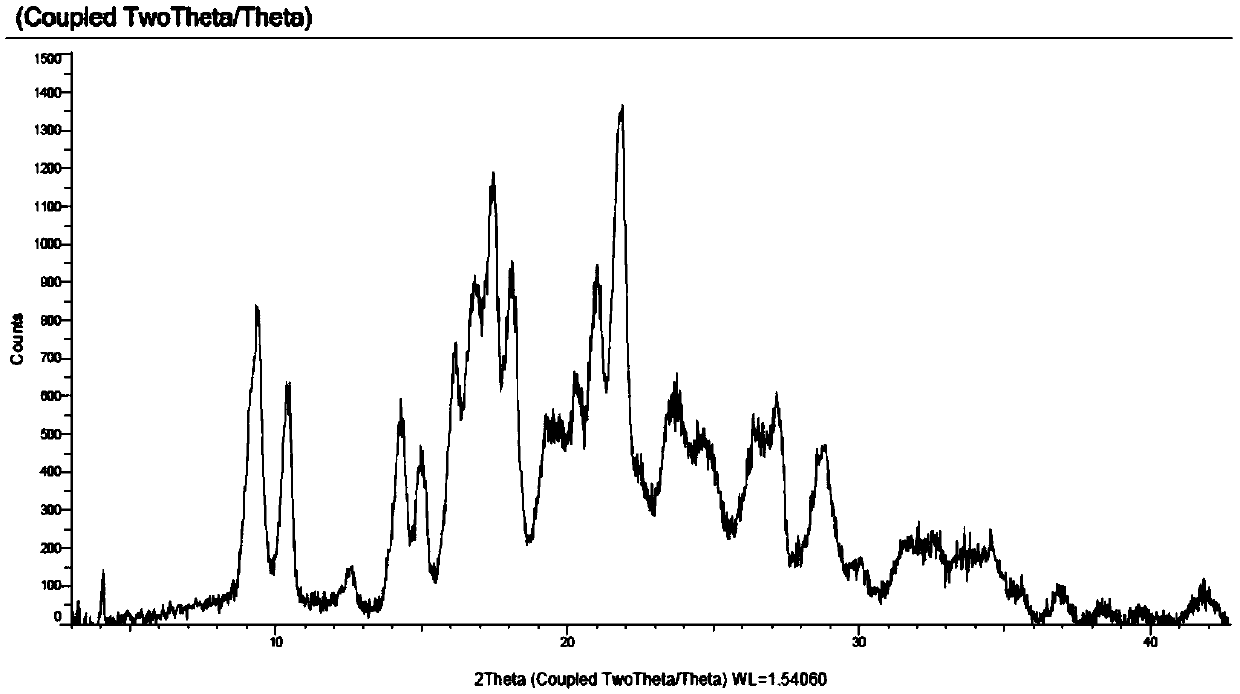
Iclaprim mesylate crystal form B and preparation method thereof
A technology of alaprime mesylate and crystal form, which is applied in the directions of sulfonate preparation, organic chemistry method, pharmaceutical formulation and the like, and achieves the effects of good stability and purity, low preparation cost and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Suspend 5g of Ilaprim mesylate (HPLC purity: 98.8%) in 60ml of ethanol, under nitrogen protection, heat up to 80°C, add 0.5g of activated carbon after dissolving, stir for 10min, filter while hot, and the filtrate is at room temperature Crystals were precipitated under stirring for 1 hour, filtered, and dried to obtain 4.2 g of the solid, whose purity by HPLC was 99.7%.
Embodiment 2
[0037] Suspend 5g of Ilaprim mesylate (HPLC purity: 98.8%) in 70ml of isopropanol, under nitrogen protection, heat up to 90°C, then add 4ml of pure water until dissolved, then add 0.5g of activated carbon, and stir for 10min , filtered while hot, and the filtrate was stirred at room temperature to precipitate crystals for 1.5 hours, filtered, and 3.7 g of solids were dried, and the purity was 99.9% by HPLC.
Embodiment 3
[0039] Suspend 5g of Ilaprim mesylate (HPLC purity: 98.8%) in 60ml of methanol, under nitrogen protection, heat up to 70°C, add 0.5g of activated carbon after dissolving, stir for 10min, filter while hot, and the filtrate is at room temperature Crystals were precipitated under stirring for 1 hour, filtered, and 4.0 g of the solid was dried, and the purity was 99.8% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com