Pharmaceutical composition for treating gastrointestinal disease and application thereof
A technology of gastrointestinal diseases and compositions, which is applied in the field of pharmaceutical compositions for the treatment of gastrointestinal diseases, can solve the problems of large adverse effects, slow onset of effects, inability to treat both symptoms and root causes, and achieve stable curative effect and not easy to repeat
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
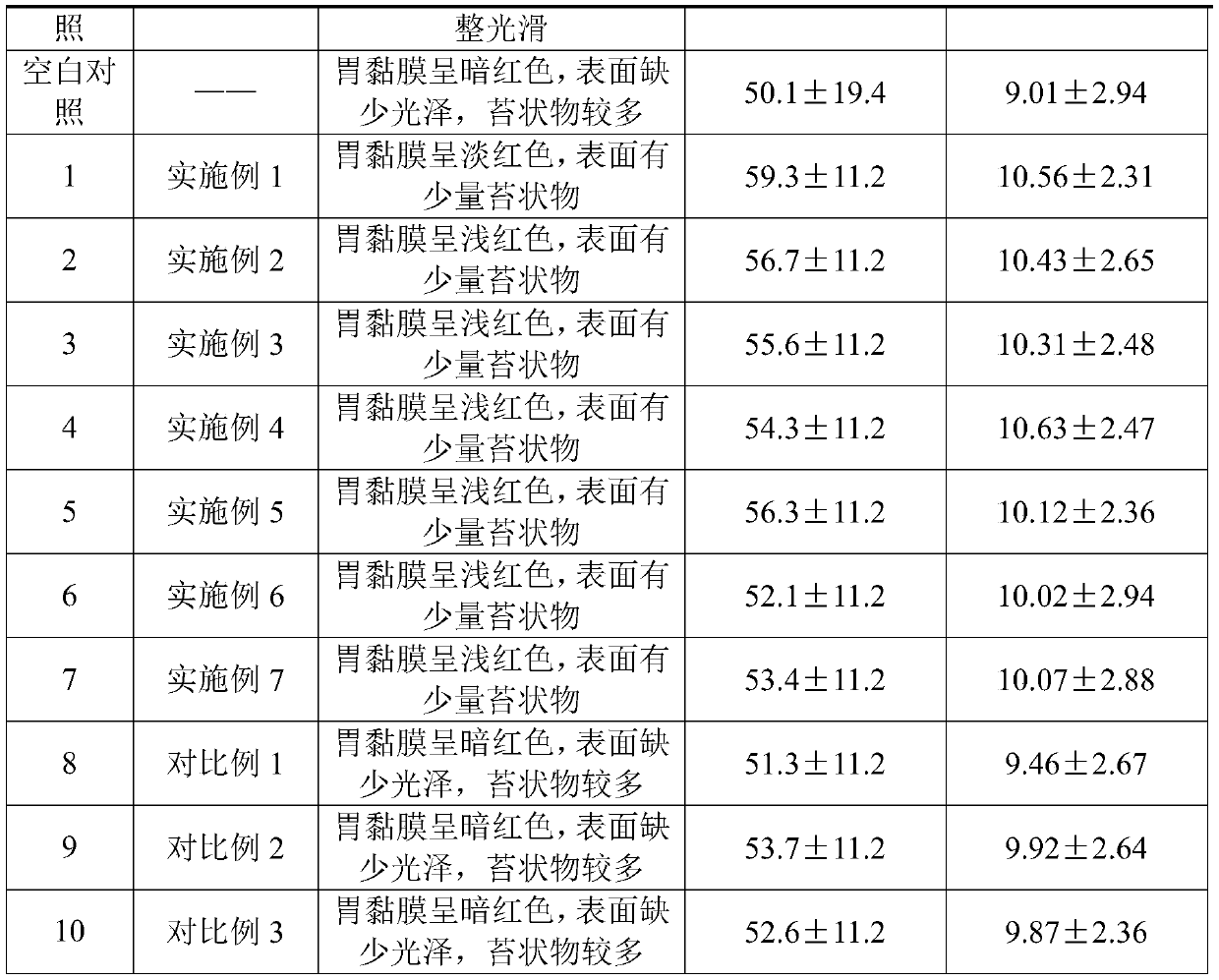
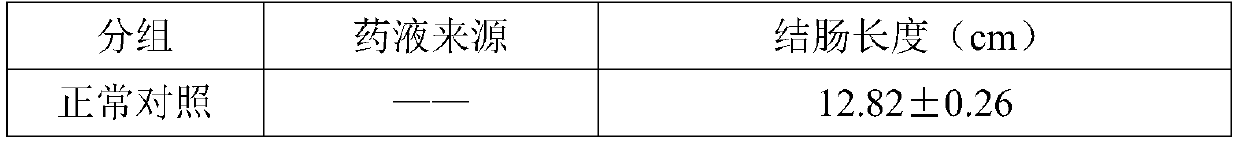
Examples
Embodiment 1
[0026] The pharmaceutical composition for the treatment of gastrointestinal diseases provided in this embodiment includes, in parts by weight, 20 parts of litsea cubeba oil, 20 parts of linseed oil, 23 parts of cloud sesame oil, 20 parts of seabuckthorn oil, 20 parts of Amomum yangchun, 10 parts of Lycium barbarum seed oil, 10 parts of Ganoderma lucidum spore oil.
Embodiment 2
[0028] The pharmaceutical composition for the treatment of gastrointestinal diseases provided by the present embodiment, in parts by weight, includes: 17 parts of litsea cubeba oil, 18 parts of linseed oil, 25 parts of cloud sesame oil, 17 parts of seabuckthorn oil, 17 parts of Amomum yangchun, 9 parts of wolfberry seed oil, 9 parts of Ganoderma lucidum spore oil.
Embodiment 3
[0030] The pharmaceutical composition for the treatment of gastrointestinal diseases provided by the present embodiment, in parts by weight, includes: 22 parts of litsea cubeba oil, 22 parts of linseed oil, 20 parts of cloud sesame oil, 23 parts of seabuckthorn oil, 23 parts of Amomum yangchun, 11 parts of wolfberry seed oil, 11 parts of Ganoderma lucidum spore oil.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com