Bromhexine hydrochloride solution preparation for inhalation and preparation method thereof
A technology of bromhexine hydrochloride and bromhexine hydrochloride, applied in the field of bromhexine hydrochloride solution preparation for inhalation and preparation thereof, can solve problems such as affecting safety, and achieve the effects of reliable quality, shortening the course of disease and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
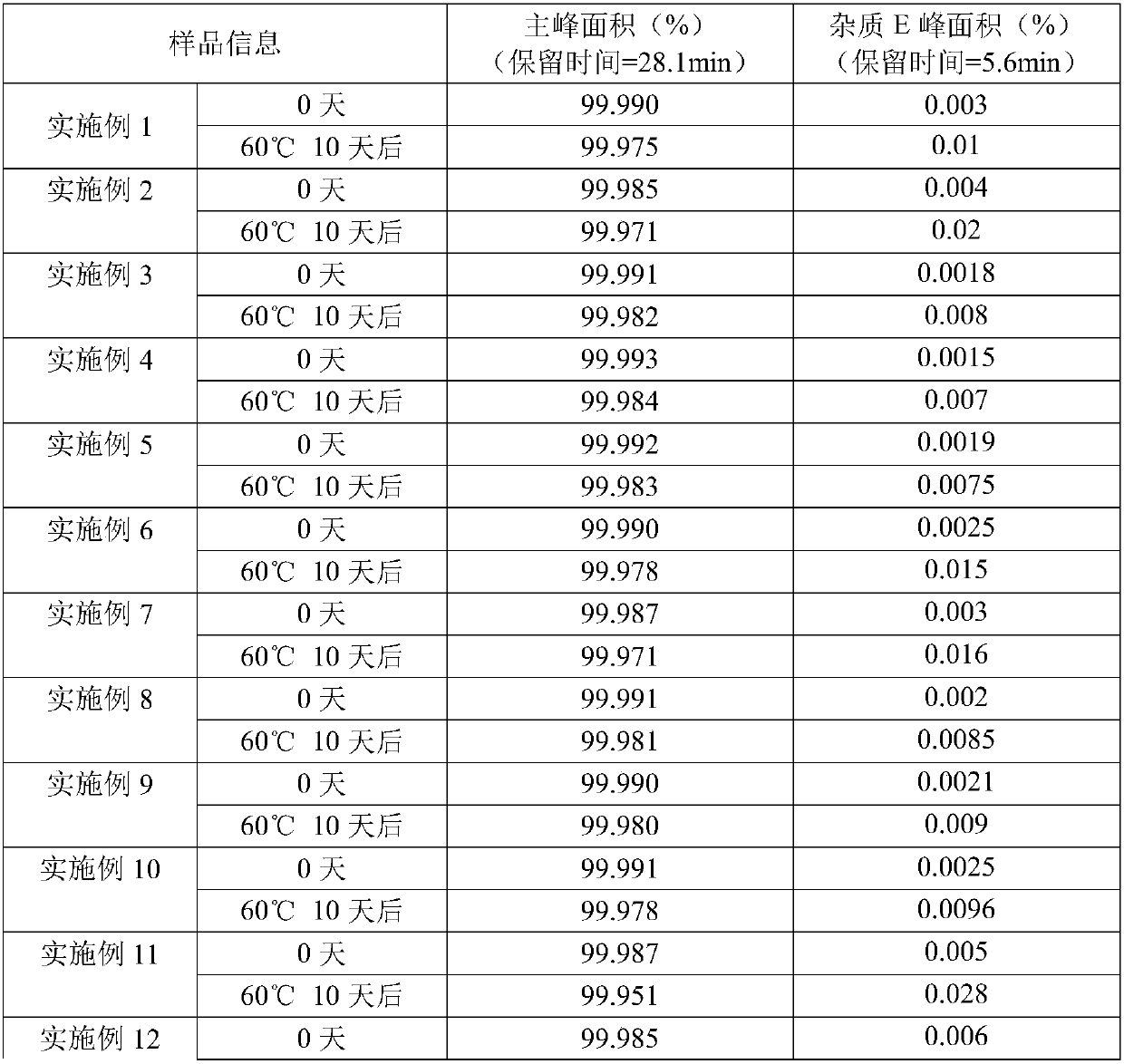
Embodiment 1-7
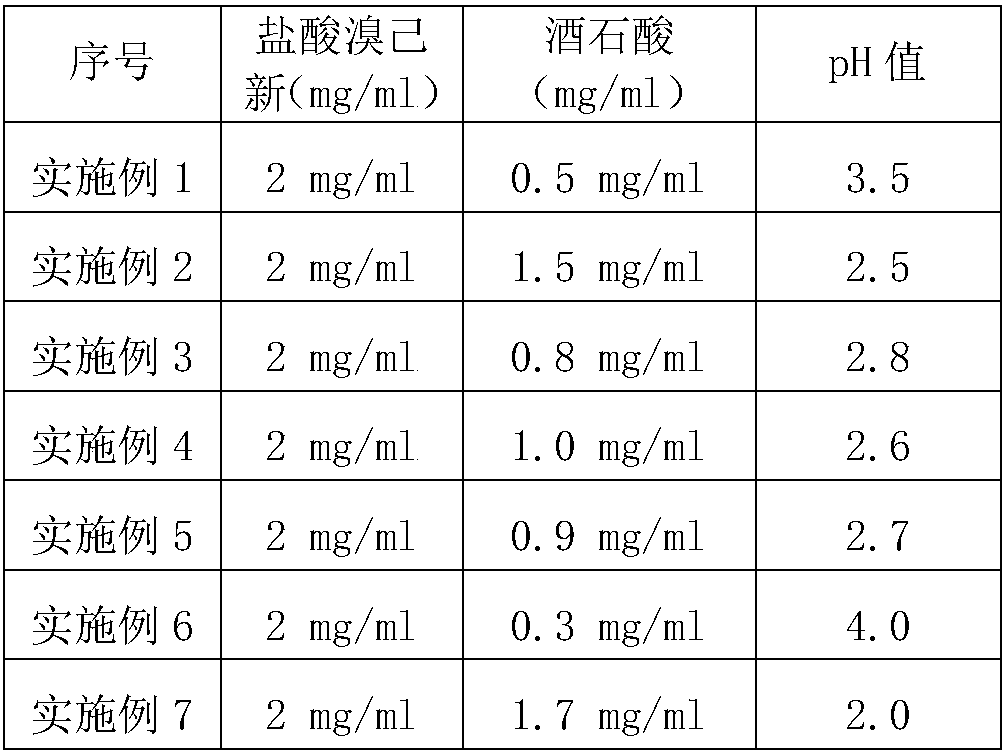
[0029] Embodiments 1-7 have the same preparation method of bromhexine hydrochloride solution preparations for inhalation, but different prescriptions. The specific dosages of bromhexine hydrochloride and tartaric acid in the prescriptions are shown in Table 1.
[0030] (1) Take the prescribed amount of tartaric acid and bromhexine hydrochloride, add them to water for injection at 50°C in turn, stir until completely dissolved, and obtain a pre-inhalation solution;
[0031] (2) The pre-inhalation solution obtained in step (1) is fine-filtered with a 0.22 μm filter membrane to obtain a bromhexine solution; before and after filling the bromhexine solution, nitrogen protection is filled in the ampoule, and under aseptic conditions Next, the bromhexine solution is filled in the ampoule and sealed, and packed to obtain the bromhexine hydrochloride solution preparation for inhalation of the present invention.
[0032] The concrete consumption of bromhexine hydrochloride, tartaric acid...
Embodiment 8
[0035] prescription
[0036] Bromhexine Hydrochloride 1.5mg / ml
[0037] Tartaric acid 0.75mg / ml
[0038] (1) Take the prescribed amount of tartaric acid and bromhexine hydrochloride, add them to deionized water at 45°C in turn, and stir until completely dissolved to obtain a pre-inhalation solution;
[0039] (2) The pre-inhalation solution obtained in step (1) is fine-filtered with a 0.22 μm filter membrane to obtain a bromhexine solution; before and after filling the bromhexine solution, nitrogen protection is filled in the ampoule, and under aseptic conditions Next, the bromhexine solution is filled in the ampoule and sealed, and packed to obtain the bromhexine hydrochloride solution preparation for inhalation of the present invention, and its pH value is 2.65.
Embodiment 9
[0041] prescription
[0042] Bromhexine Hydrochloride 3mg / ml
[0043] Tartaric acid 0.9mg / ml
[0044] (1) Take the prescribed amount of tartaric acid and bromhexine hydrochloride, add them to distilled water at 58°C in turn, and stir until completely dissolved to obtain a pre-inhalation solution;
[0045] (2) The pre-inhalation solution obtained in step (1) is fine-filtered with a 0.22 μm filter membrane to obtain a bromhexine solution; before and after filling the bromhexine solution, nitrogen protection is filled in the ampoule, and under aseptic conditions Next, the bromhexine solution is filled in the ampoule and sealed, and packed to obtain the bromhexine hydrochloride solution preparation for inhalation of the present invention, and its pH value is 2.6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com