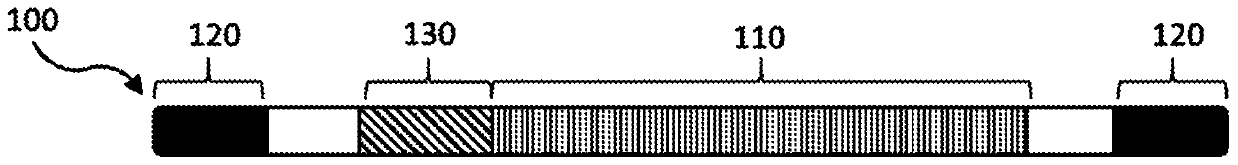
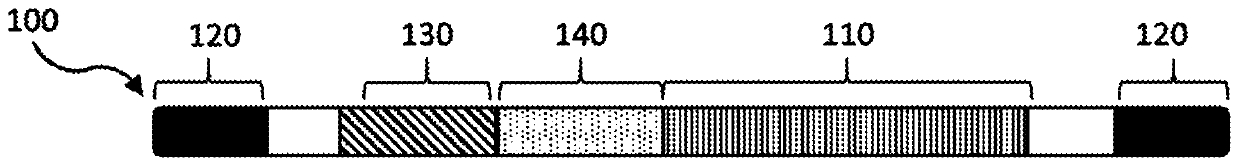
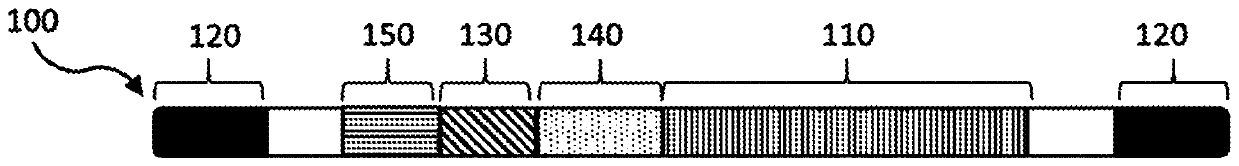
Compositions and methods of treating amyotrophic lateral sclerosis (ALS)
A technology of SOD1 and vectors, applied in biochemical equipment and methods, drug combinations, gene therapy, etc., can solve the problems of inability to prolong the survival of SOD1 mice, prolong the survival of neurons, and insufficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0799] Formulations of the pharmaceutical compositions described herein can be prepared by any method known or later developed in the art of pharmacology. Generally, such preparation methods include the steps of bringing the active ingredient into association with excipients and / or one or more other auxiliary ingredients, and then, if necessary and / or desired, separating, shaping and / or packaging the product into the desired Single-dose or multiple-dose units as desired.
[0800] The relative amounts of the active ingredients, the pharmaceutically acceptable excipients and / or any other ingredients in the pharmaceutical composition according to the invention will vary depending on the nature, size and / or condition of the subject to be treated, and It further depends on the route by which the composition is to be administered.
[0801] AAV particles comprising regulatory polynucleotide sequences encoding siRNA molecules of the invention can be formulated with one or more excipi...
Embodiment 1
[0982] Embodiment 1.SOD1 siRNA design, synthesis and analysis
[0983] SOD1 siRNA design
[0984] siRNA design was performed to identify siRNAs targeting the human SOD1 gene. The design uses the human-specific SOD1 transcript (Genebank Accession NM_000454.4 (SEQ ID NO: 1254)).
[0985] The siRNA duplexes were designed to have 100% identity with the human SOD1 transcript for positions 2-18 of the antisense strand and partial OR with non-human primate SOD1 transcript for positions 2-18 of the antisense strand. 100% identity. In all siRNA duplexes, position 1 of the antisense strand was engineered to be a U, and position 19 of the sense strand was engineered to be a C, in order to unpair the duplex at this position.
[0986] Selection and synthesis of SOD1 siRNA sequences
[0987] Based on the predicted selectivity of the antisense strand for the human, cynomolgus and rhesus SOD1 genes, and the lack of a match between the seed sequence at positions 2-7 of the antisense ...
Embodiment 2
[0994] Example 2. SOD1 siRNA needles selected in SH-SY5Y cells, U87 cells and primary human astrocytes In vitro screening for expression of endogenous SOD1 mRNA
[0995] SH-SY5Y cells were obtained from ATCC (ATCC in partnership with LGC Standards, Wesel, Germany) and grown in a humidified incubator at 37°C, 5% CO 2 Under an atmosphere supplemented with 15% FCS (ultra-low IgG from GIBCO / Life Technologies), 1% L-glutamine (Biochrom GmbH, Berlin, Germany) and 1% penicillin / streptomycin (Biochrom GmbH, Berlin, Germany) ) in Dulbecco's MEM (Biochrom GmbH, Berlin, Germany).
[0996] U87MG cells were obtained from ATCC (ATCC in partnership with LGC Standards, Wesel, Germany) and grown in a humidified incubator at 37°C, 5% CO 2 ATCC-prepared Eagle's Minimal Basal Medium (compared to LGC Standards Partner ATCC, Wesel, Germany).
[0997] Primary human astrocytes were obtained from LONZA (Lonza Sales Ltd, Basel, Switzerland) and grown in a humidified incubator at 37°C, 5% CO 2 C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com