Recombinant human B-type natriuretic peptide injection and preparation method thereof
A natriuretic peptide and injection technology, applied in the field of pharmaceutical inventions, can solve problems such as poor solubility and excessive bubbles, and achieve good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Formula: recombinant human B-type natriuretic peptide 0.6mg / ml, citric acid 8mg / ml, sodium chloride 2mg / ml, activated carbon 0.2mg / ml, and the balance is water for injection.
[0047] Embodiment 1 is prepared by any of the following preparation methods:
[0048] Preparation method 1
[0049] 1) Turn on the chilled water system, add cooled water for injection into the liquid distribution tank, cool down to 12°C, close the chilled water system, and start stirring;
[0050] 2) adding recombinant human B-type natriuretic peptide under stirring;
[0051] 3) Slowly add sodium chloride and citric acid to control the pH value to 5.1-5.5;
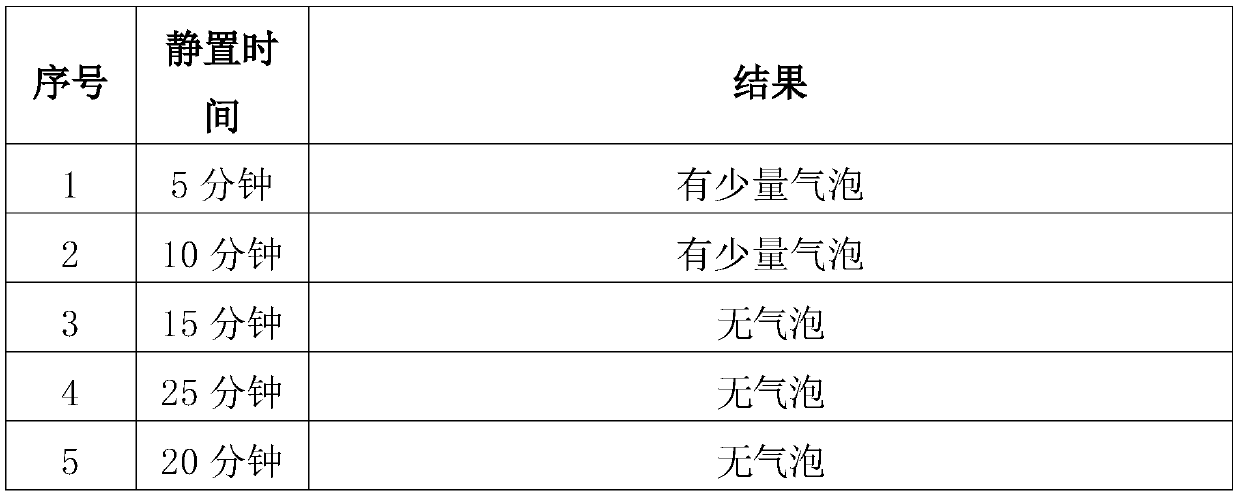
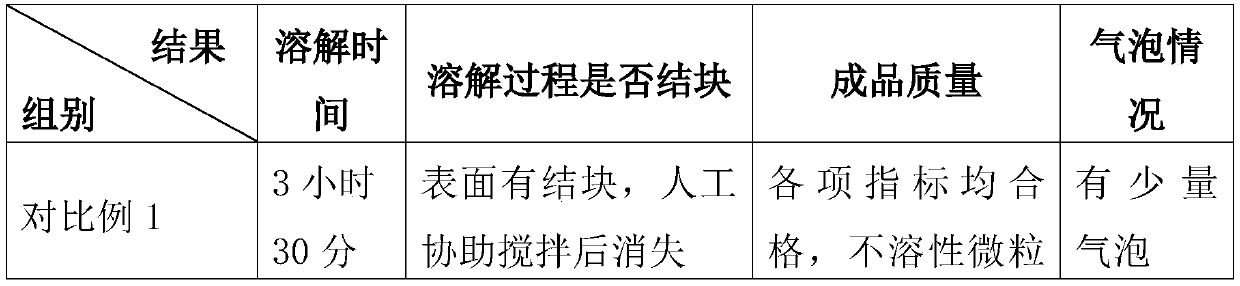
[0052] 4) Add activated carbon, add water for injection to the total volume of the solution, continue to stir for 15 minutes to pre-dissolve, stop stirring, and let stand for 15 to 25 minutes;
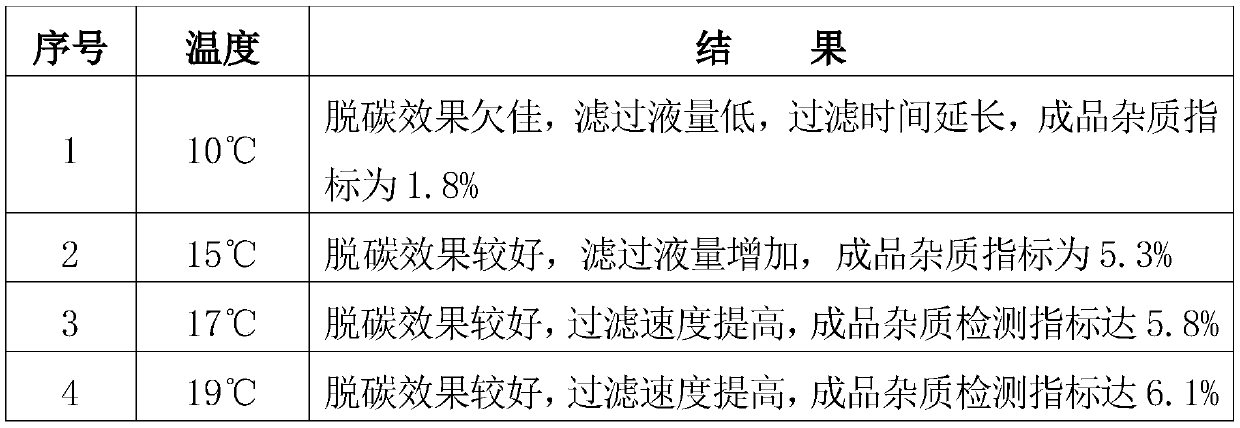
[0053] (5) Simultaneously carry out decarburization at a temperature of 15°C and pass through a sterilizing filter with a pore size of 0.22um for on...
Embodiment 2
[0079] Formula: recombinant human B-type natriuretic peptide 1.0mg / ml, citric acid 11mg / ml, sodium chloride 5mg / ml, activated carbon 0.5mg / ml, and the balance is water for injection.
[0080] Embodiment 2 is prepared by any of the following preparation methods:
[0081] Preparation method 1
[0082] 1) Turn on the chilled water system, add cooled water for injection into the liquid distribution tank, cool down to 12°C, close the chilled water system, and start stirring;
[0083] 2) adding recombinant human B-type natriuretic peptide under stirring;
[0084] 3) Slowly add sodium chloride and citric acid to control the pH value to 5.1-5.5;
[0085] 4) Add activated carbon, add water for injection to the total volume of the solution, continue to stir for 15 minutes to pre-dissolve, stop stirring, and let stand for 15 to 25 minutes;
[0086] (5) Simultaneously carry out decarburization at a temperature of 15°C and pass through a sterilizing filter with a pore size of 0.22um for...
Embodiment 3
[0112] Formula: recombinant human B-type natriuretic peptide 0.7mg / ml, citric acid 8.5mg / ml, sodium chloride 2.5mg / ml, activated carbon 0.2mg / ml, and the balance is water for injection.
[0113] Embodiment 3 is prepared by any of the following preparation methods:
[0114] Preparation method 1
[0115] 1) Turn on the chilled water system, add cooled water for injection into the liquid distribution tank, cool down to 12°C, close the chilled water system, and start stirring;
[0116] 2) adding recombinant human B-type natriuretic peptide under stirring;
[0117] 3) Slowly add sodium chloride and citric acid to control the pH value to 5.1-5.5;
[0118] 4) Add activated carbon, add water for injection to the total volume of the solution, continue to stir for 15 minutes to pre-dissolve, stop stirring, and let stand for 15 to 25 minutes;
[0119] (5) Simultaneously carry out decarburization at a temperature of 15°C and pass through a sterilizing filter with a pore size of 0.22um ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap