Application of transthyretin in transferring fusion protein into eyes
A technology of thyroxine protein and protein, which is applied in the application field of transthyretin carrying exogenous protein to the eye through the eye barrier, which can solve the problems of short half-life and low delivery efficiency, and achieve good biocompatibility and safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of transthyretin: comprising the following steps:
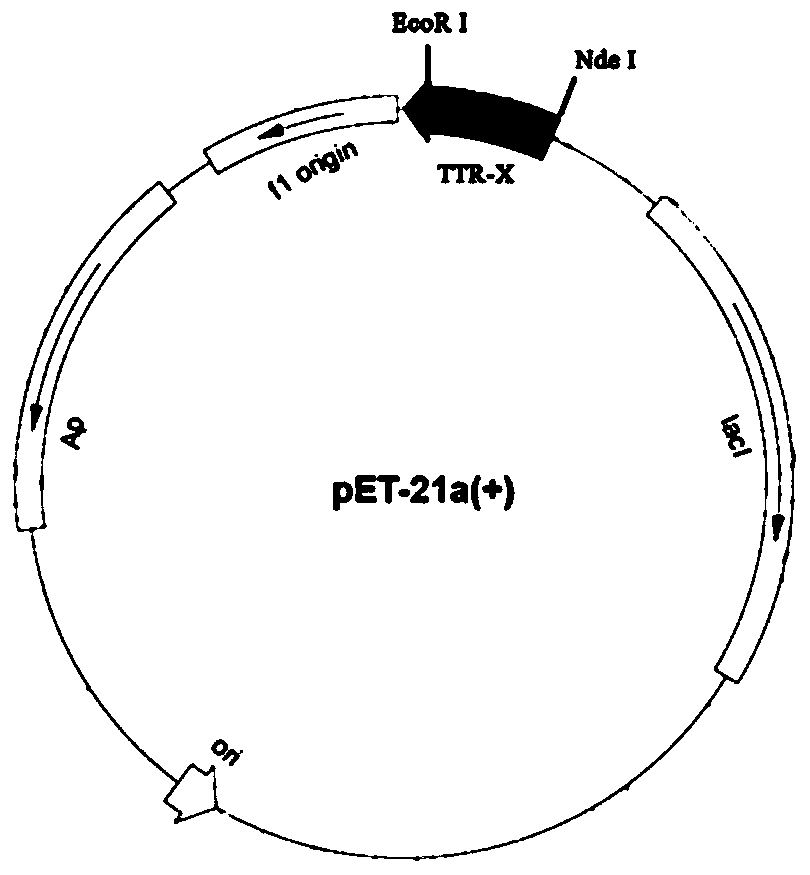
[0031] (1) Construction of recombinant plasmid pET 21a(+)-TTR: Synthesize the nucleotide sequence shown in SEQ ID NO.2, connect it to pET 21a(+) with Nde I and EcoR I enzymes, and verify by sequencing , build succeeds.
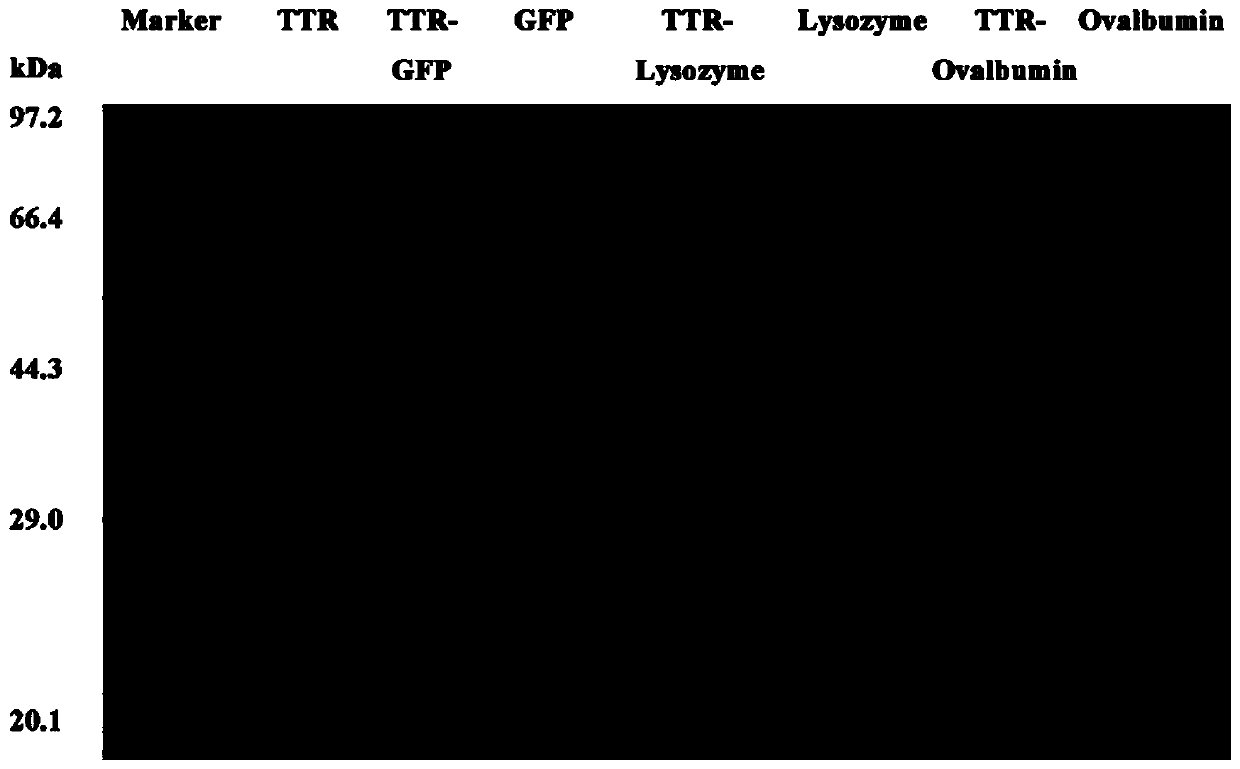
[0032] (2) Expression and purification of recombinant TTR: transform the pET 21a(+)-TTR plasmid constructed in step (1) into E.coliBL21(DE3) cells, and culture recombinant E.coli BL21(DE3) in LB medium , prepare the seed solution, and then insert it into 5L TB medium with a 5% inoculum amount, at a temperature of 37°C, with a stirring paddle speed of 150rpm, and cultivate to OD 600 1.5-2.0; add 0.1-0.5mM IPTG to induce 8-16h (Table 1). The TTR was prepared by high-pressure homogenization and the supernatant was subjected to affinity adsorption on a nickel column. The resulting protein was passed through an endotoxin adsorption column (Pierce TM High Capacity Endotoxin Removal SpinColum...
Embodiment 2
[0036] The fusion protein of transthyretin-green fluorescent protein (TTR-GFP) was prepared according to the following method:
[0037] (1) Construction of recombinant plasmid pET 21a(+)-TTR-GFP: Synthesize the TTR-GFP sequence shown in SEQ ID NO.3, connect it to pET 21a(+) with Nde I and EcoR I enzymes, and pass Sequencing verification, the construction was successful.
[0038](2) Expression of TTR and GFP fusion protein: Transform the recombinant plasmid constructed in step (1) into E.coli BL21 (DE3) cells of pET 21a (+)-TTR-GFP plasmid with a 5% inoculation amount, insert 5L TB medium, temperature 37°C, stirring paddle speed 150rpm, culture OD 600nm to 1.5-2.0; add 0.1-0.5mM IPTG to induce for 8-16h; use high pressure homogeneously break the bacteria and prepare the TTR-GFP fusion protein by nickel column affinity adsorption on the supernatant (Table 2). The resulting protein was passed through an endotoxin adsorption column (Pierce TM High Capacity Endotoxin Removal Sp...
Embodiment 3
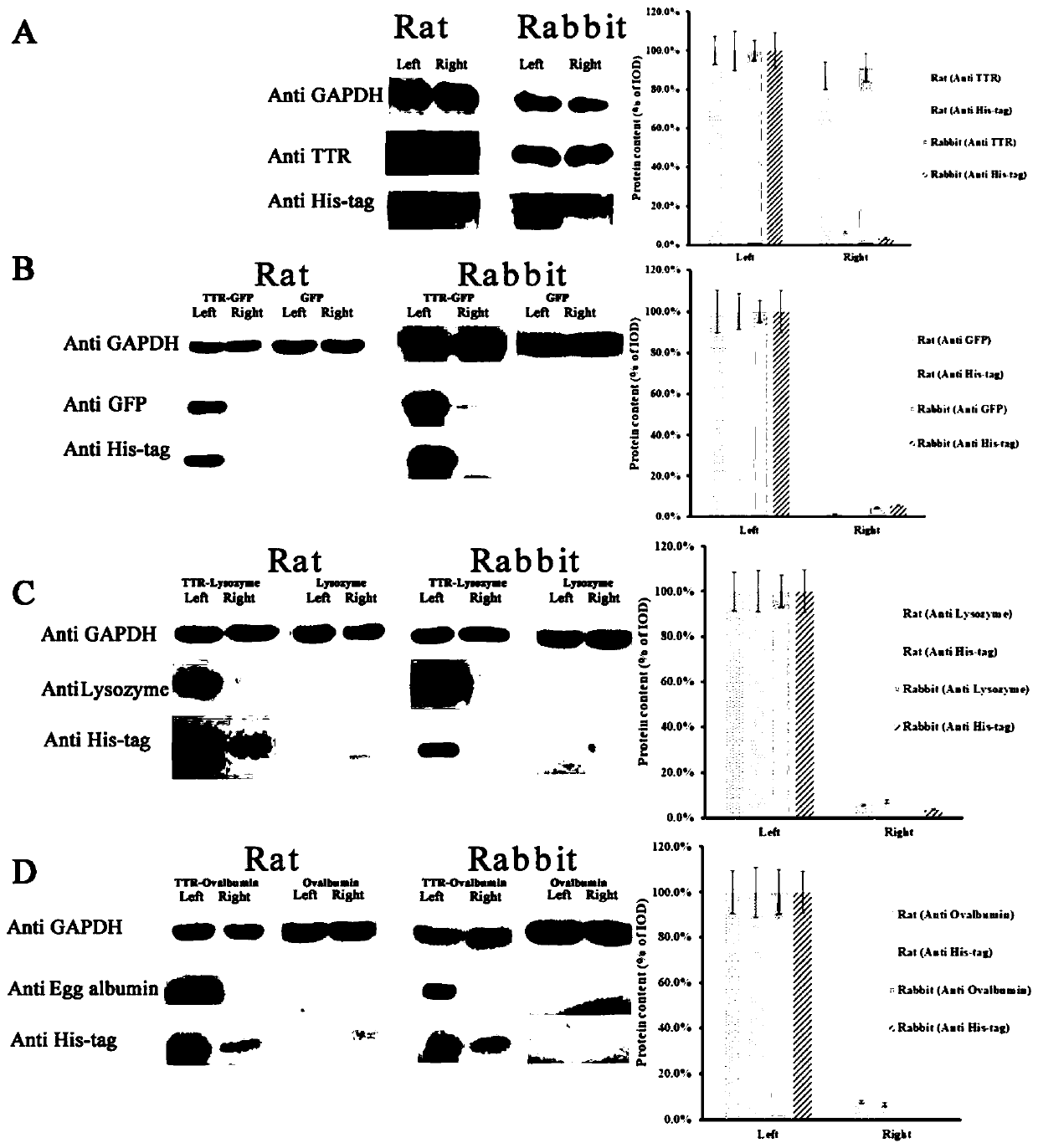
[0043] A method for fusion expressing transthyretin to transport exogenous protein into the eye through the eye barrier, the method comprising the following steps:
[0044] (1) Construction of recombinant plasmid pET 21a(+)-TTR-Lysozyme: synthesize the TTR-Lysozyme sequence shown in SEQ ID NO.4, connect it to pET 21a(+) with Nde I and EcoR I enzymes, and Sequencing verification, the construction was successful.
[0045] (2) Transform the pET 21a(+)-TTR-Lysozyme constructed in step (1) into E.coli BL21(DE3) cells with a 5% inoculum amount, insert 5L TB medium at 37°C, and stirrer speed 150rpm, culture OD 600nm to 1.5-2.0; add 0.1-0.5mM IPTG to induce for 8-16h; use high pressure homogenization to break the bacteria and prepare the TTR-Lysozyme fusion protein by nickel column affinity adsorption on the supernatant (Table 3). The resulting protein was passed through an endotoxin adsorption column (Pierce TM HighCapacity Endotoxin Removal Spin Columns, ThermoFisher) to remove ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com