Industrial method for clean recovery of olanzapine mother solution
A olanzapine parenteral and clean technology, applied in the direction of organic chemistry, can solve the problems of environmental pollution, waste of economic value of olanzapine, and no recycling of organic solvents, so as to reduce environmental pollution, reduce emissions, and extremely The effect of economic value and social value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
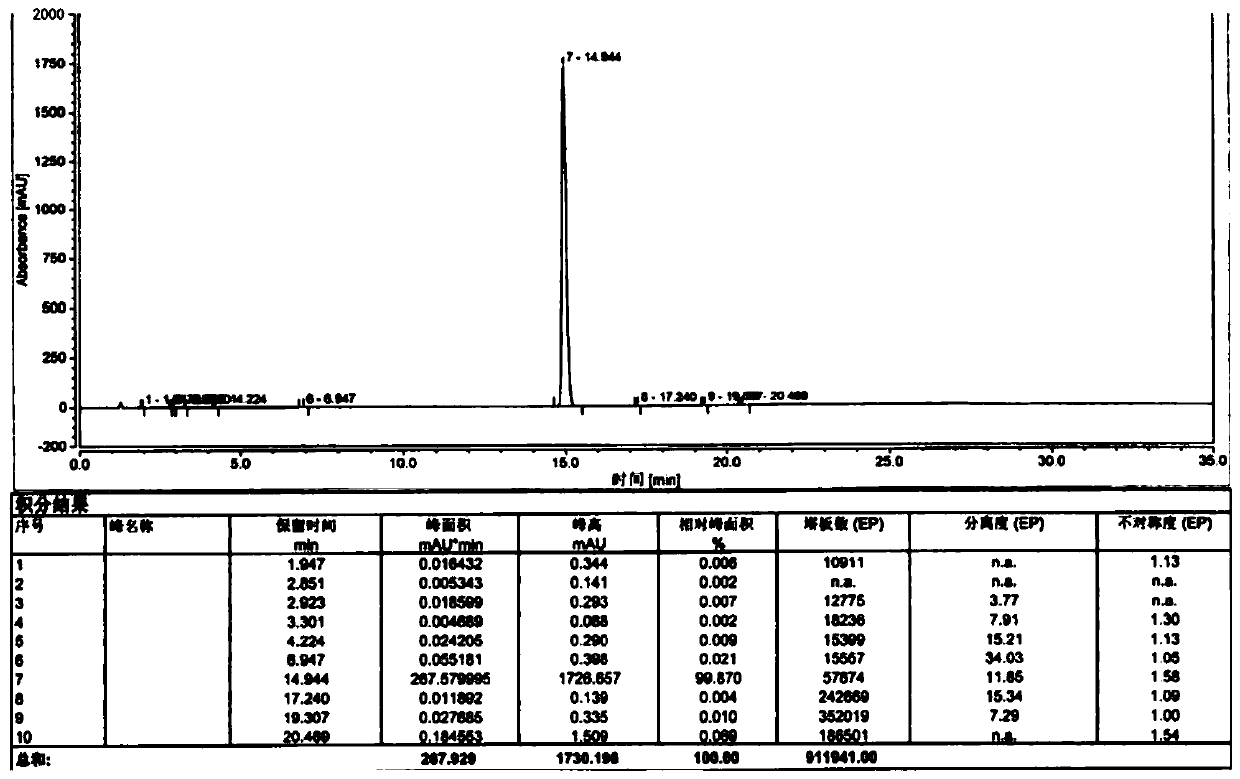
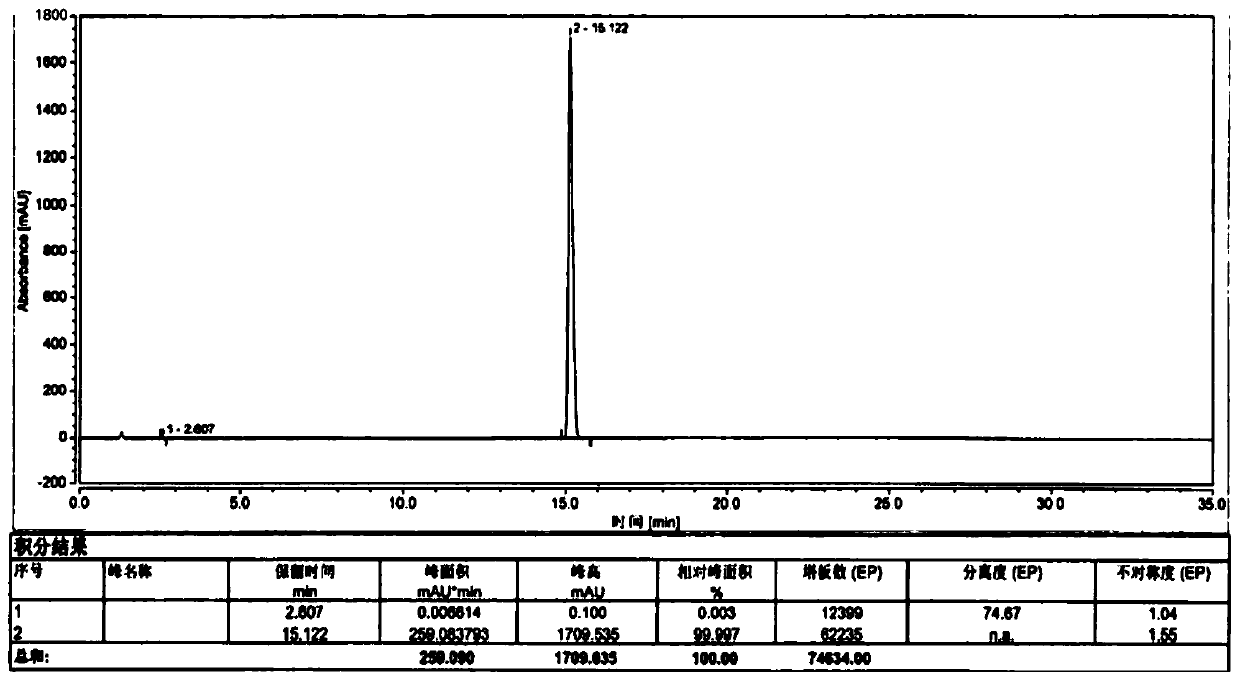
[0040] Referring to the preparation method of patent CN1179160A, add 20kg olanzapine crude product and 200L ethyl acetate into the reaction tank, heat up and dissolve, cool down and crystallize, and get about 200L olanzapine mother liquor after rejection filtration, and obtain 14.5kg olanzapine after drying the filter cake Flat, yield 72.5%;
[0041] Transfer the olanzapine mother liquor into the distillation tank, heat up and distill to recover the solvent ethyl acetate, stop the recovery when the solid precipitates in the tank, recover about 140L of ethyl acetate, the content is 99.3%, and the recovery rate is 70.0%, and then decompress and dry the tank inner material;
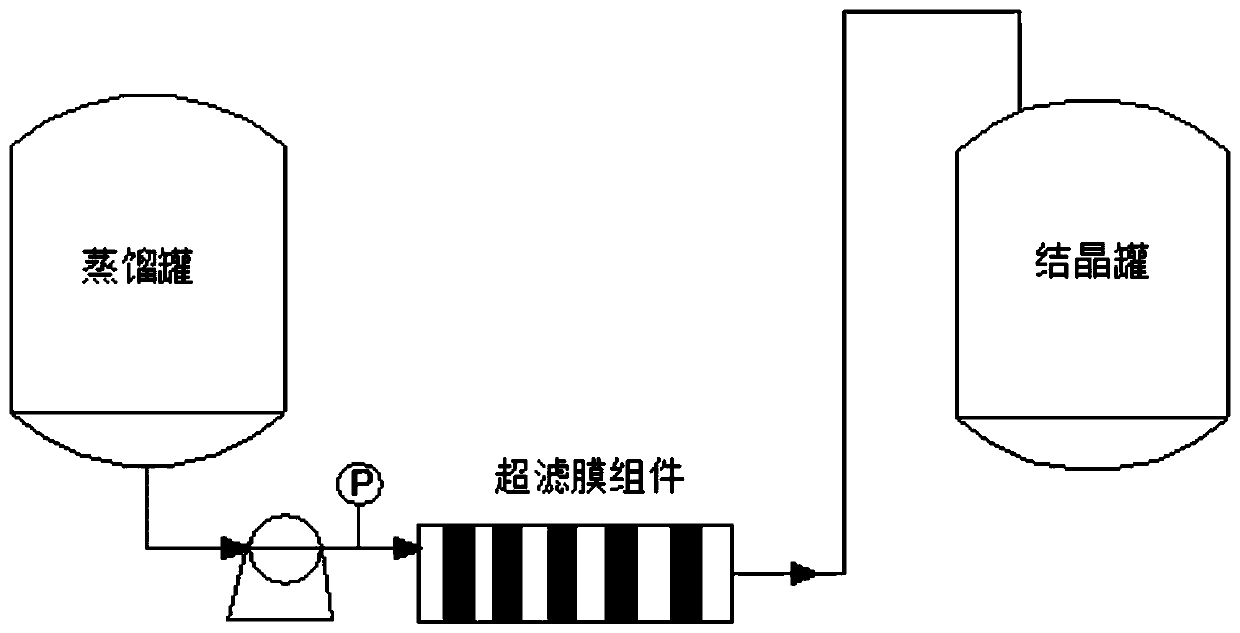
[0042] Add 60kg of water into the tank, start stirring, adjust the pH value to 3.0 with hydrochloric acid, heat up to 40°C to dissolve, and obtain a clear and transparent liquid through the ultrafiltration membrane module, transfer it to the crystallization tank, and oxidize it with 10% hydrogen The pH valu...
Embodiment 2
[0044] Referring to the preparation method of patent CN1466588A, add 20kg olanzapine crude product and 90L dichloromethane into the reaction tank, add activated carbon to decolorize after heating up and dissolving, crystallize after pressure filtration, obtain about 70L olanzapine mother liquor after rejection filtration, and dry the filter cake Afterwards, 12.5kg olanzapine was obtained, with a yield of 62.5%;
[0045] Transfer the olanzapine mother liquor into a distillation tank, heat up and distill to recover the solvent dichloromethane, stop the recovery when solids are precipitated in the tank, recover about 48L of dichloromethane, the content is 99.5%, and the recovery rate is 53.3%, and then decompress and dry the tank inner material;
[0046] Add 80kg of water into the tank, start stirring, adjust the pH value to 4.0 with acetic acid, heat up to 40°C to dissolve, and obtain a clear and transparent liquid through the ultrafiltration membrane module, transfer it to the ...
Embodiment 3
[0048] With reference to the preparation method of patent CN1420117A, 20kg olanzapine crude product and 200L ethanol were added into the reaction tank, after heating up and dissolving, activated carbon was added for decolorization, crystallization after pressure filtration, about 180L of olanzapine mother liquor was obtained after rejection filtration, and the filter cake was dried to obtain 15.9kg olanzapine, yield 79.5%;
[0049] Transfer the olanzapine mother liquor into the distillation tank, heat up and distill to recover the solvent ethanol, stop the recovery when solids are precipitated in the tank, recover about 152L of ethanol, with a content of 99.2%, and a recovery rate of 76.0%, and then decompress and dry the material in the tank;
[0050] Add 50kg of water into the tank, start stirring, adjust the pH value to 5.0 with acetic acid, heat up to 40°C to dissolve, and obtain a clear and transparent liquid through the ultrafiltration membrane module, transfer it to the ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap