Application of ADORA1 in preparation of PD-L1/PD-1 monoclonal antibody tumor immunotherapy drug
A technology of PD-L1 and anti-tumor immunity, which is applied in the field of biology, PD-L1/PD-1 monoclonal antibody tumor immunotherapy, and can solve problems such as unclear mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
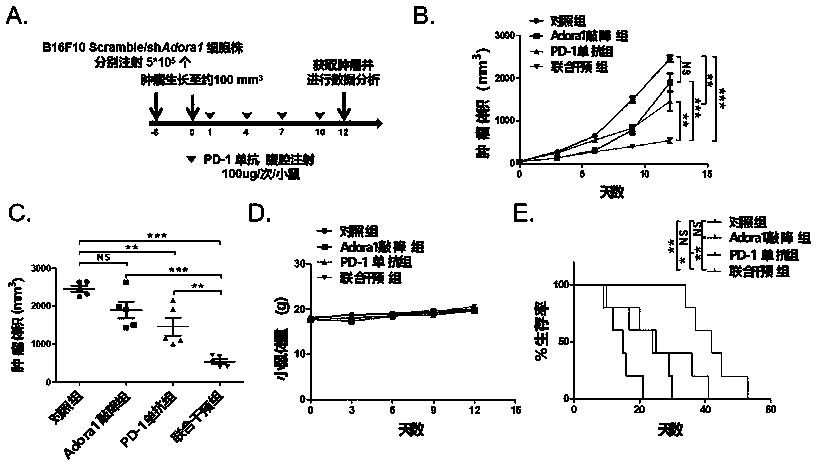
[0038] 1. In vivo study of gene knockdown adenosine receptor ADORA1 combined with PD-1 monoclonal antibody against proliferation of transplanted tumors (melanoma) in mice.
[0039] 1.1 Group settings:
[0040] Scramble+IgG2a (control group)
[0041] shAdora1+IgG2a (Adora1 knockdown group)
[0042] Scramble+PD-1mAb (PD-1 monoclonal antibody treatment group)
[0043] shAdora1+PD-1mAb (combined intervention group)
[0044] 1.2 Experimental procedure, see figure 1 A:
[0045] About 6 days before the experiment: 6-8 weeks C57BL / 6 mice were injected subcutaneously into the right dorsal wing of B16F10 melanoma shAdora1 knockdown cell line 5*10 5 20 mice each and the same number of Scramble cell lines (control group).
[0046] Experiment day 0: Record the mouse body weight and tumor volume, when the tumor volume grows to about 100mm 3 At the beginning of the hour, mice inoculated with shAdora1 knockdown cell line and Scramble cell line were injected with PD-1 mAb 100ug / mouse / 3 days respectively, a...
Embodiment 2
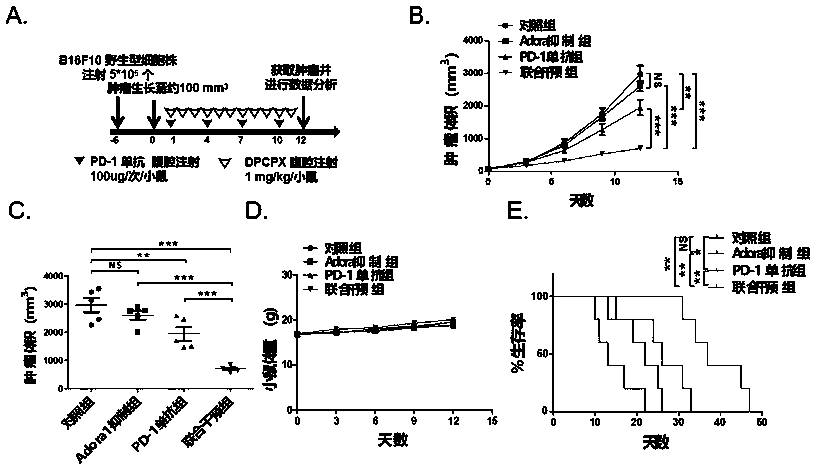
[0062] 1. The in vivo study of the specific inhibitor DPCPX targeting the adenosine receptor ADORA1 combined with PD-1 monoclonal antibody against the proliferation of mouse transplanted tumors (melanoma, non-small cell lung cancer).
[0063] 1.1 Group settings:
[0064] Vehicle+IgG2a (control group)
[0065] DPCPX+IgG2a (Adora1 inhibition group)
[0066] Vehicle+PD-1mAb (PD-1 monoclonal antibody treatment group)
[0067] DPCPX+PD-1mAb (combined intervention group)
[0068] 1.2 Experimental procedure, see image 3 A, 4A:
[0069] About 6 days before the experiment: 6-8 weeks of C57BL / 6 mice were injected subcutaneously into the right dorsal wing of B16F10 melanoma 5*10 5 Or LLC non-small cell lung cancer 1*10 6 A wild-type cell line, 40 mice each.
[0070] Experiment day 0: Record the mouse body weight and tumor volume, when the tumor volume grows to about 100mm 3 At the beginning of the hour, the vaccinated mice were divided into four groups, received DPCPX 1mg / Kg / mouse, intraperitoneal i...
Embodiment 3
[0092] 1. In vivo study of gene knockdown transcription factor ATF3 combined with adenosine receptor ADORA1 specific inhibitor DPCPX against proliferation of transplanted tumors (melanoma, non-small cell lung cancer) in mice.
[0093] 1.1 Group settings:
[0094] Scramble+vehicle (control group)
[0095] Scramble+DPCPX (Adora1 suppression group)
[0096] ShAtf3+vehicle (Atf3 knock-down group)
[0097] ShAtf3+DPCPX (joint intervention group)
[0098] 1.2 Experimental procedure, see Figure 5 A, 6A:
[0099] About 6-8 days before the experiment: 6-8 weeks C57BL / 6 mice were injected subcutaneously into the right dorsal wing of B16F10 melanoma shAtf3 knockdown cell line 5*10 5 Or LLC non-small cell lung cancer shATF3 knockdown cell line 1*10 6 One, and the same number of Scramble cell lines (control group), each with 20 mice.
[0100] Experiment day 0: Record the mouse body weight and tumor volume, when the tumor volume grows to about 100mm 3 At the beginning of the hour, mice inoculated with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com