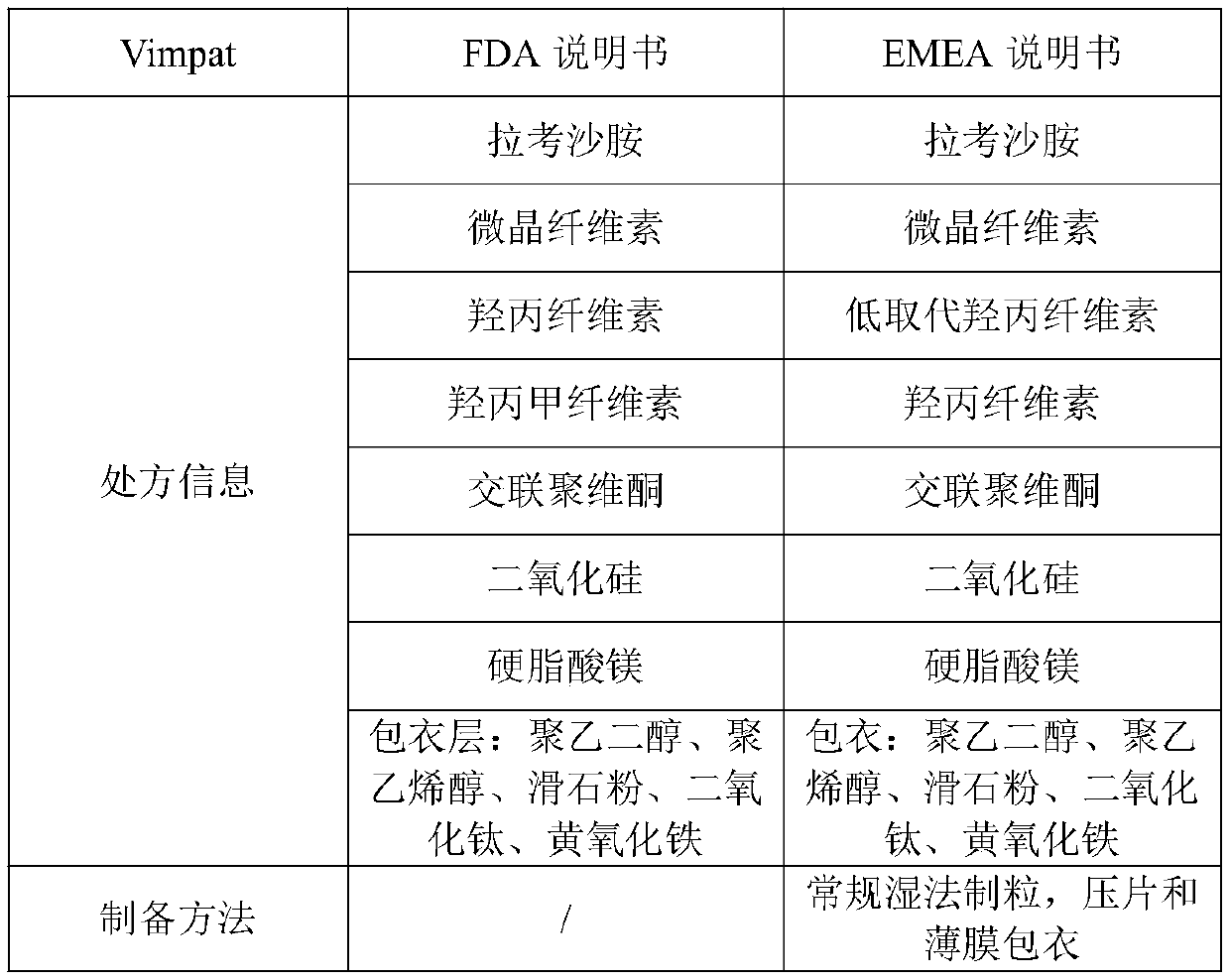
Preparation method of lacosamide tablet
A technology of lacosamide and salamine tablets, which is applied in the direction of non-active ingredient medical preparations, pharmaceutical formulas, active ingredients of amides, etc., can solve the problems of poor dissolution uniformity, reduce dissolution, and reduce intra-batch variation , Improve the effect of dissolution uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
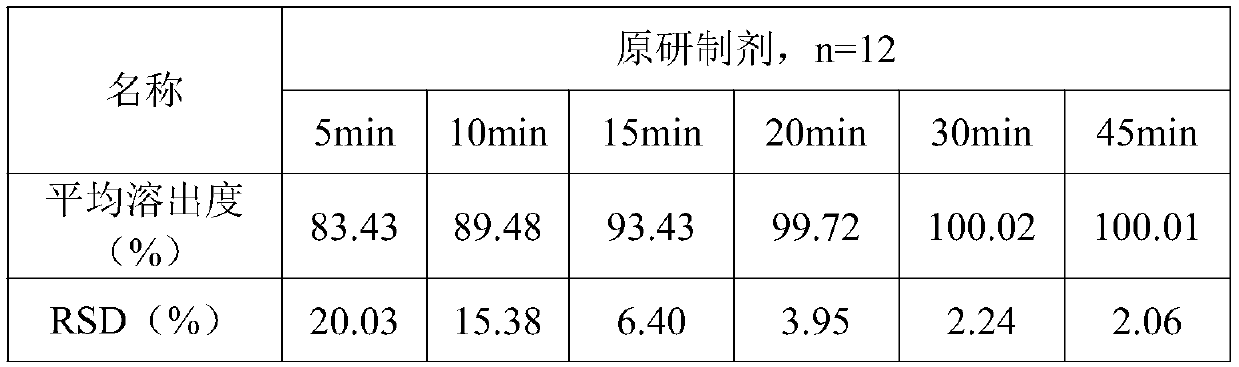
[0066] Example 1: Effects of Different Preparation Methods on the Dissolution Uniformity of the Same Prescription Tablet
[0067] According to the following prescriptions, 1000 tablets were prepared by different methods, and the influence of different preparation methods on the dissolution uniformity of the same prescription tablets was investigated.
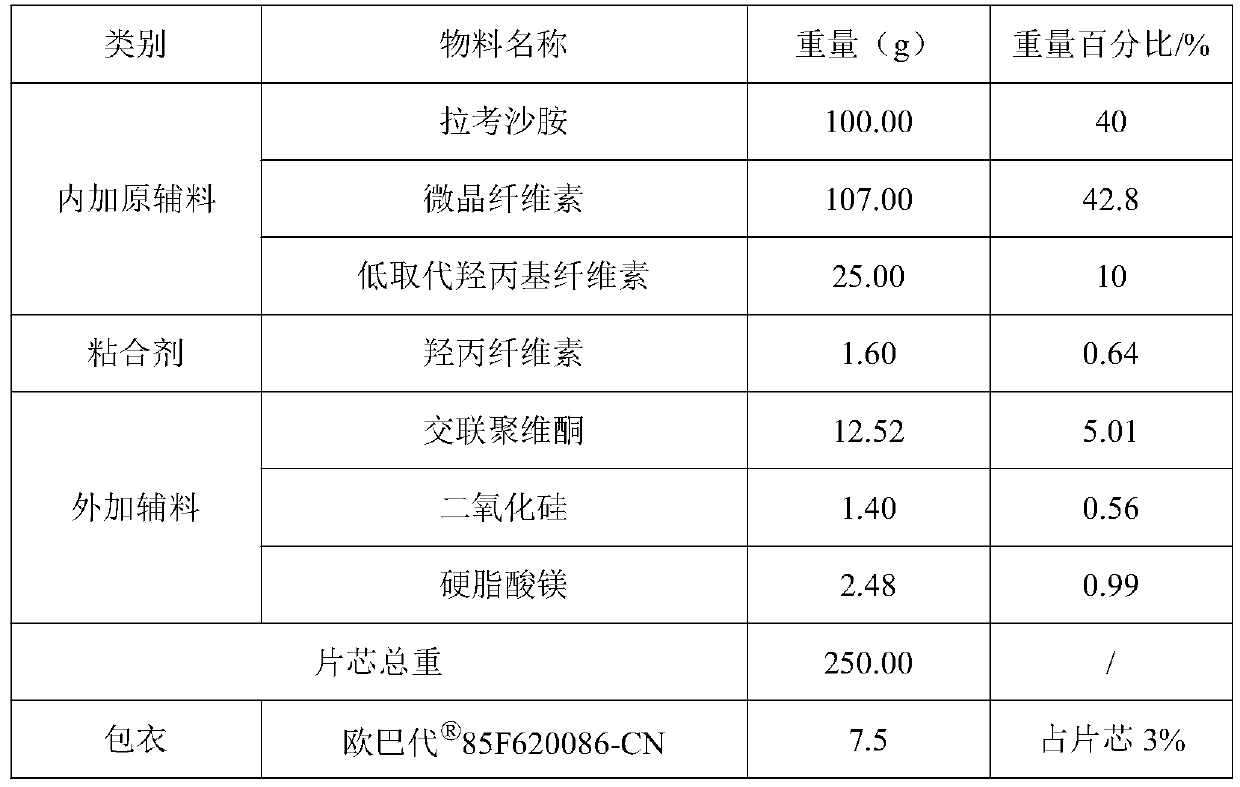
[0068] 1. Embodiment 1 prescription is as follows:
[0069] Table 2 Example 1 prescription
[0070]
[0071] 2. The preparation method is as follows:
[0072] Method A:
[0073] (1) Preparation of raw and auxiliary materials: crush the raw materials and pass through an 80-mesh sieve. Lacosamide, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, hydroxypropyl cellulose, crospovidone, silicon dioxide, and magnesium stearate were weighed in prescription quantities.
[0074] (2) Put lacosamide, microcrystalline cellulose, and low-substituted hydroxypropyl cellulose in a wet mixing granulator, stir at 100 rp...
Embodiment 2
[0102] Embodiment 2: The effect of binder adding ratio in batches on dissolution uniformity
[0103] Adopt the process method of method C in embodiment 1, only change the addition ratio of adhesive twice (see Table 5 for the specific ratio, the percentage that accounts for the adhesive prescription amount), other process conditions are exactly the same, prepare 1000 pieces of tablets agent. Each group took 12 tablet samples respectively, and adopted the same method as in Example 1 to detect the dissolution rate. The results are shown in Table 6, and the results of the dissolution uniformity are shown in Table 7.
[0104] Table 5 Different ratios of binders
[0105] Example Binder for mixing in step (2) Step (3) water-soluble adhesive 2-1 30% 70% 2-2 40% 60% 2-3 50% 50% 2-4 60% 40% 2-5 70% 30%
[0106] Table 6 Dissolution Data
[0107]
[0108] Table 7 Dissolution Uniformity Data
[0109]
[0110] As can be seen from Tabl...
Embodiment 3
[0111] Embodiment 3: the influence of different excipients on dissolution uniformity
[0112] Using different tablet prescriptions, 1000 tablets were prepared by the preparation process of Example 2-3, and 12 tablets were taken respectively, and the dissolution rate was detected by the same method as in Example 1. The specific prescription is shown in Table 8-10, and the dissolution results are shown in Table 11-12.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com