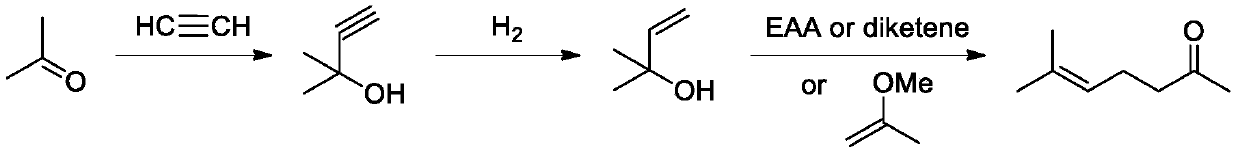
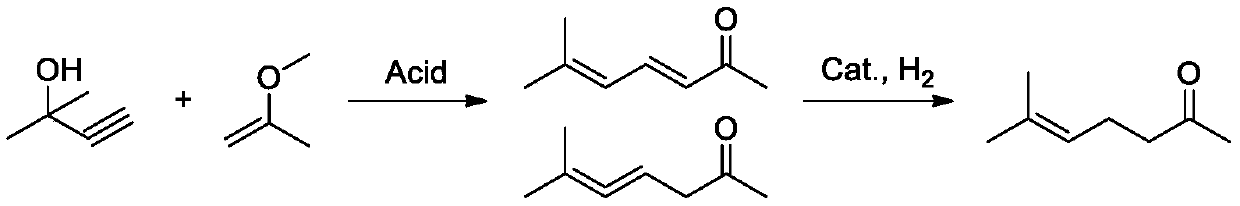
Method for synthesizing methyl heptenone from methyl butynol
A technology of methyl butynol and methyl heptenone, applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problem of slow reaction speed, low production efficiency, and inability to achieve chemical selectivity and other problems, to achieve the effect of high chemical selectivity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] At room temperature, first add sulfonic acid resin T211 (2.009g) and aluminum trichloride (2.103g) into a 500mL autoclave, seal the autoclave, and slowly replace the air in the autoclave with nitrogen for 3 times, and then use an advection pump to sequentially pump the methyl Butynol (42.058g, 0.5mol) and 2-methoxypropene (108.159g, 1.5mol) were added to the kettle. Nitrogen was added to the autoclave subsequently, and the pressure in the autoclave rose to 1.0 MPa (to prevent the vaporization of 2-methoxypropene at high temperature). Turn on stirring and heating, when the internal temperature of the reactor rises to 60°C, start timing, keep the constant temperature reaction, take samples and analyze regularly, and monitor the progress of the reaction by GC. After 3 hours, GC showed that the conversion rate of the raw material methyl butynol was 89.3%, and the total selectivity of the products diketenone and methylheptadienone was 98.3%. The catalyst was filtered off, a...
Embodiment 2
[0039] At room temperature, first add sulfonic acid resin T211 (0.210g) and aluminum trichloride (0.210g) into a 500mL autoclave, seal the autoclave, and slowly replace the air in the autoclave with nitrogen for 3 times, and then use a convection pump to sequentially pump the methyl Butynol (42.058g, 0.5mol) and 2-methoxypropene (72.106g, 1.0mol) were added to the kettle. Nitrogen is then added to the autoclave, and the pressure in the autoclave rises to 2.0 MPa (to prevent the vaporization of 2-methoxypropene at high temperature). Turn on stirring and heating, and when the internal temperature of the reactor rises to 70°C, start timing, keep the constant temperature reaction, take samples and analyze regularly, and monitor the progress of the reaction by GC. After 2 hours, GC showed that the conversion rate of the raw material methyl butynol was 99.7%, and the total selectivity of the product alkenone and methylheptadienone was 97.6%. The catalyst was filtered off, and the r...
Embodiment 3
[0043] At room temperature, first add sulfonic acid resin T211 (42 mg) and aluminum trichloride (42 mg) into a 500 mL autoclave, seal the autoclave, and slowly replace the air in the autoclave with nitrogen for 3 times, and then use an advection pump to sequentially inject methylbutyne Alcohol (42.058g, 0.5mol) and 2-methoxypropene (72.106g, 1.0mol) were added to the kettle. Nitrogen was added to the autoclave subsequently, and the pressure in the autoclave rose to 3.0 MPa (to prevent the vaporization of 2-methoxypropene at high temperature). Turn on stirring and heating, when the internal temperature of the reactor rises to 60°C, start timing, keep the constant temperature reaction, take samples and analyze regularly, and monitor the progress of the reaction by GC. After 3 hours, GC showed that the conversion rate of the raw material methyl butynol was 85.4%, and the total selectivity of the product alkenone and methylheptadienone was 96.9%. The catalyst was filtered off, an...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap