Fibronectin binding domain chimeric antigen receptors and methods of use thereof
A chimeric antigen receptor, fibronectin technology, used in immunoglobulins, antibody medical components, peptide/protein components, etc., can solve problems such as poor, incorrect folding, instability and flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0318] Example 1: Identification of BCMA-specific FN3 domain polypeptides
[0319] Two FN3 domain libraries totaling 25 billion variants were expressed on the surface of M13 phage and stored as phage stocks in IX PBS at 4°C. Each library stock has a titer of 1.5x10 13 cfu / ml.
[0320] The target protein BCMA-Fc chimera (R&D Systems) was bound to Protein A Dynabeads (Invitrogen) at the following concentrations: round 1: 500 nM; round 2: 200 nM; round 3: 40 nM. IgG Fc protein (R&D Systems) was bound to Protein A beads at 500 nM for all rounds as a subtractive screen. Each library was processed as a separate reaction, thus three selections were performed. The supernatant containing Fc-cleared conjugates was transferred to BCMA protein A beads. For round 1, add 1x10 for each library 13 cfu / ml of phage, and for each subsequent round, use 1x10 12 cfu / ml of phage. Beads were washed 6 times and BCMA-bound FN3 domain phage particles were eluted with low pH buffer. Phage were...
Embodiment 2
[0327] Example 2: Cloning of Chimeric Antigen Receptors Specific for Cancer Antigens
[0328] A combinatorial FN3 domain library of natural variants was generated as described in US Patent No. 8,680,019. Libraries of fibronectin binding domains will be constructed in phagemid vectors. Phage will be incubated with biotinylated cancer antigens (eg VEGF(121)) pre-bound to streptavidin-coated magnetic beads in PBS-2.5% non-fat dry milk for 1 hour at room temperature. After washing with PBS-0.1% Tween-20, bound phages were eluted with 0.1N HCl and propagated in TG1 cells for the next round of selection. In round 1, a VEGF concentration of 1 [mu]M was used and was reduced 5-fold each round so that the final round of selection contained 2OnM VEGF. To measure relative enrichment, phage prepared from non-displaying control phagemids (pBC), which confer resistance to chloramphenicol, were also included in the selection reaction. In each round, library phage are expected to be enriche...
Embodiment 3
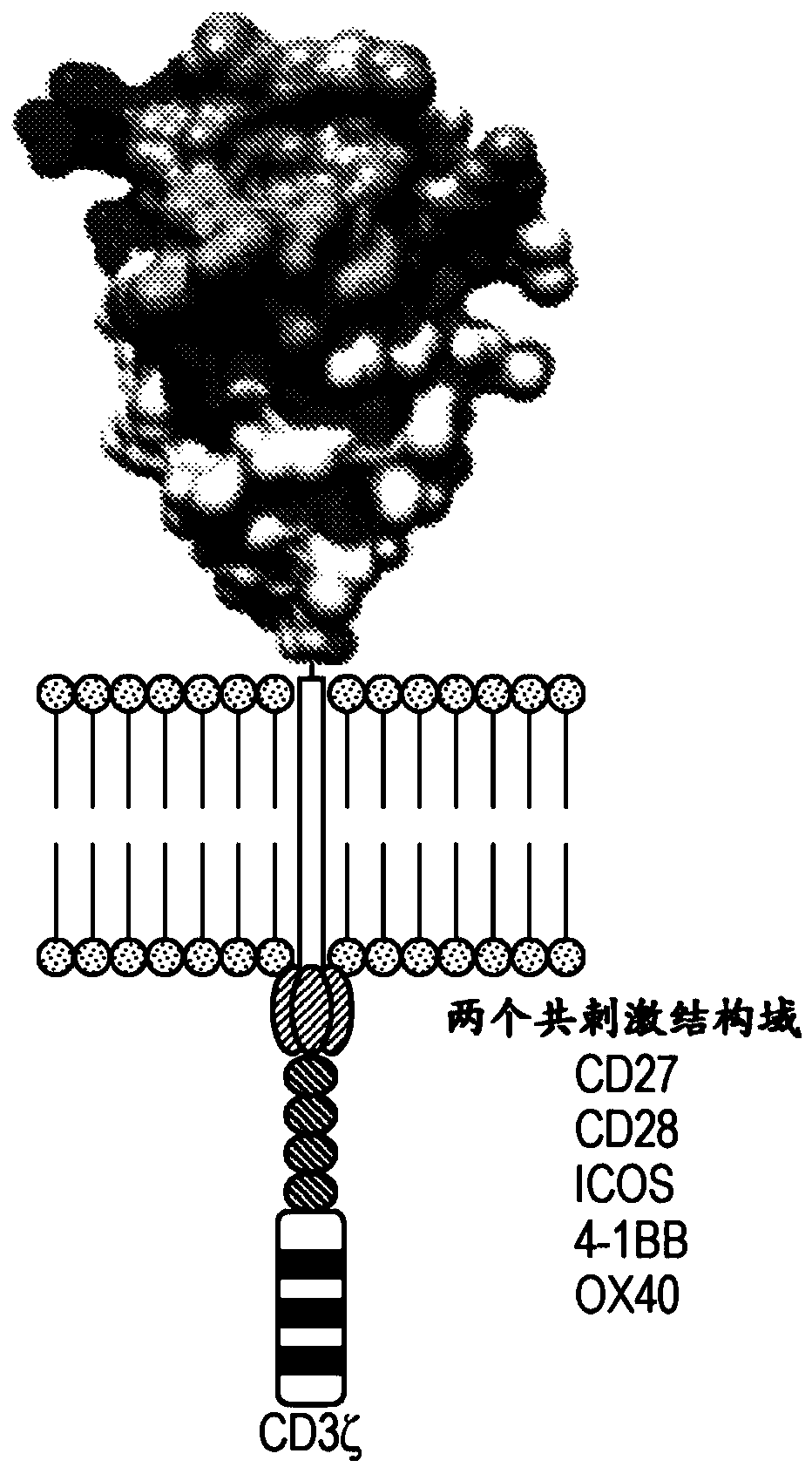
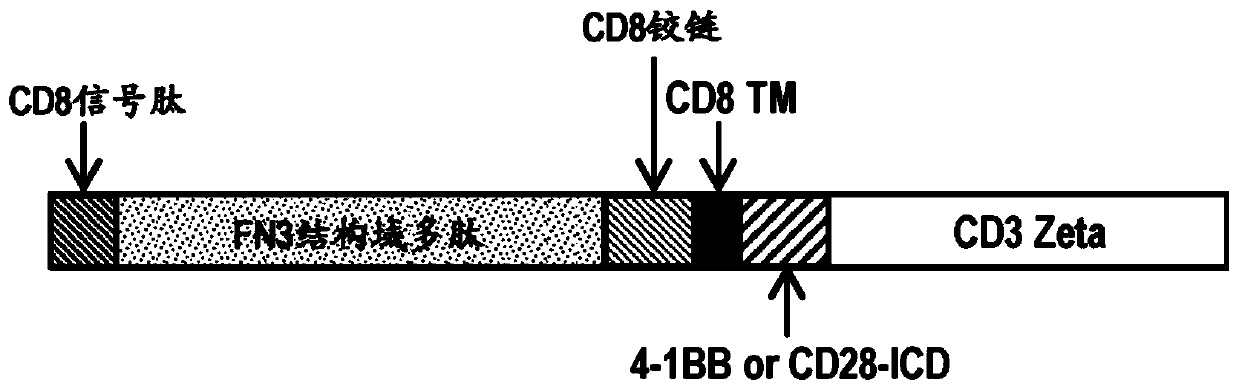
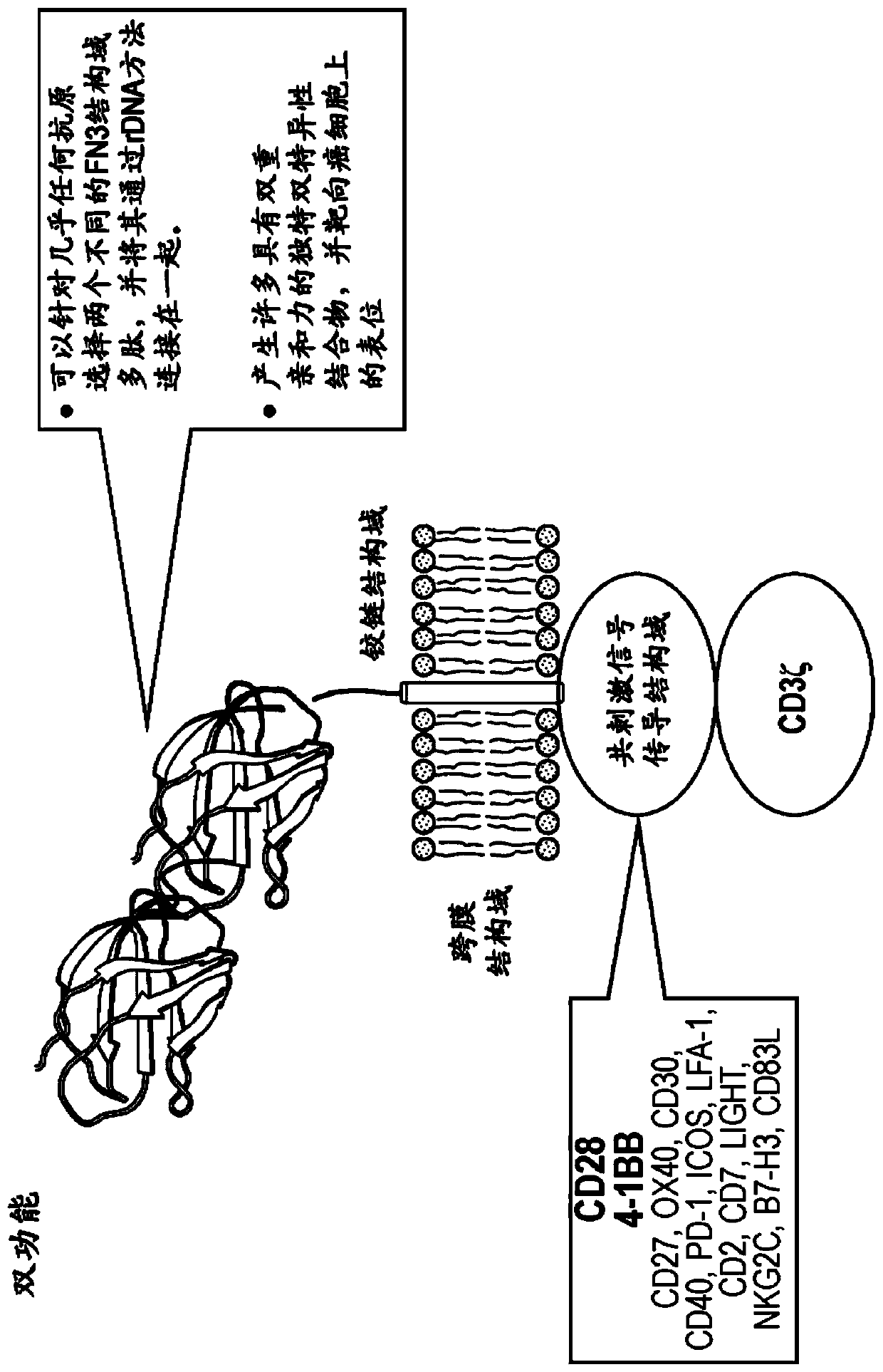
[0333] Example 3: Bifunctional FN3 domain polypeptide CAR-T
[0334] A bispecific CAR-T comprising a FN3 domain polypeptide can be constructed that expresses: i. a FN3 domain polypeptide that binds a tumor antigen, ii. a FN3 domain polypeptide that binds a T cell surface receptor, and iii. a linker. Potential tumor antigen targets are BCMA and CD-19. BCMA is involved in blood cancers, such as multiple myeloma and leukemia, and may contribute to cell survival and cell proliferation. CD19 is found on B cell cancers. Two T cell receptors that can be targeted with FN3 domain polypeptides are PD-1 and CTLA-4. Both receptors are expressed on activated T cells and transmit both inhibitory and co-stimulatory signals.
[0335] Another example of a bispecific CAR-T that includes an FN3 domain polypeptide can be constructed, including: i. a FN3 domain polypeptide that binds to AXL, ii. a FN3 domain polypeptide that binds to a T cell surface receptor or a T cell surface receptor A bod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com