Usnic acid derivatives, preparation method thereof and application thereof in preparation of anti-senile dementia drugs
A derivative, the technology of usnic acid, applied in the application field of usnic acid derivatives and its preparation, preparation and treatment of anti-senile dementia drugs, which can solve problems such as stupor and sluggishness of organisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
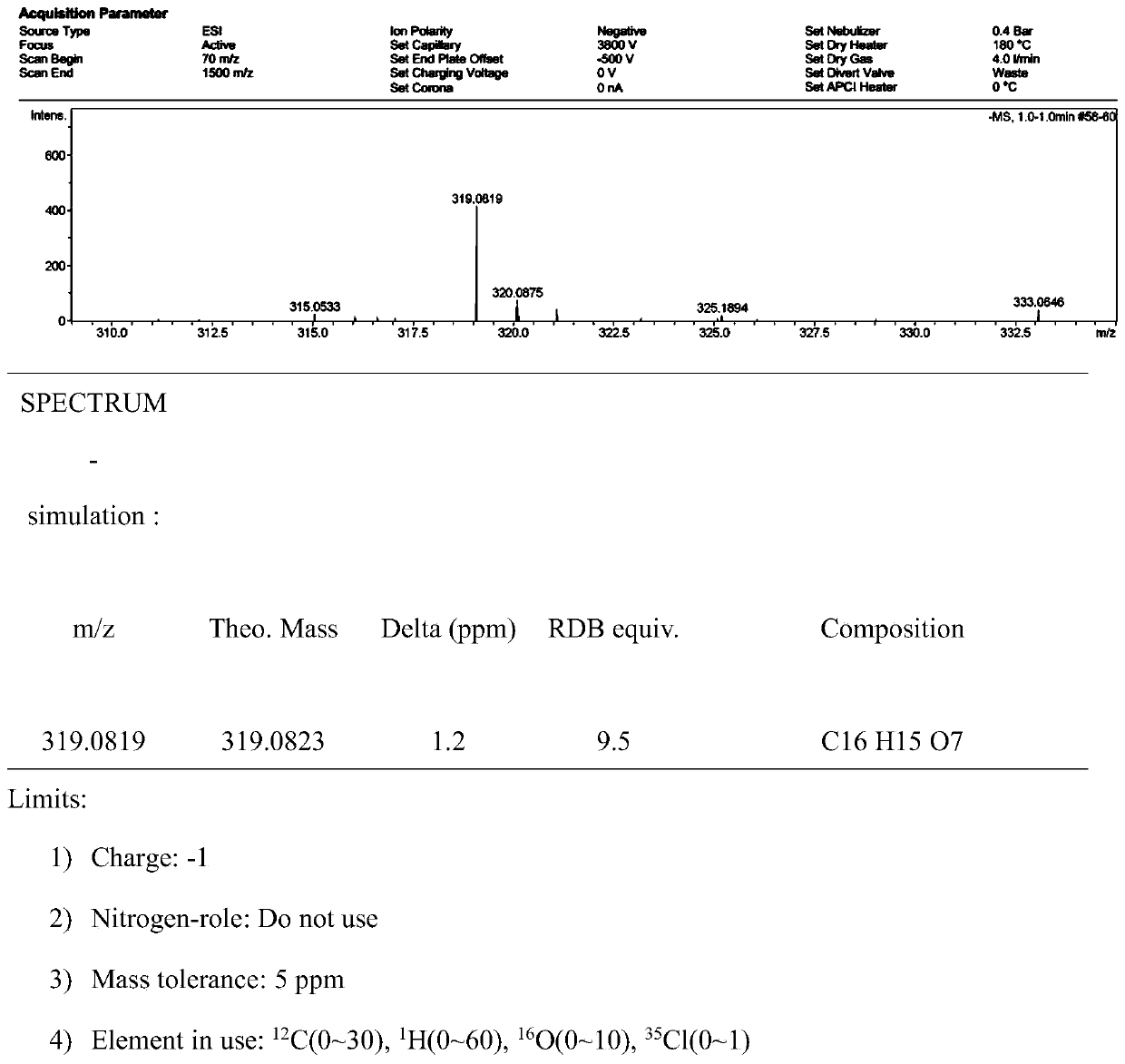
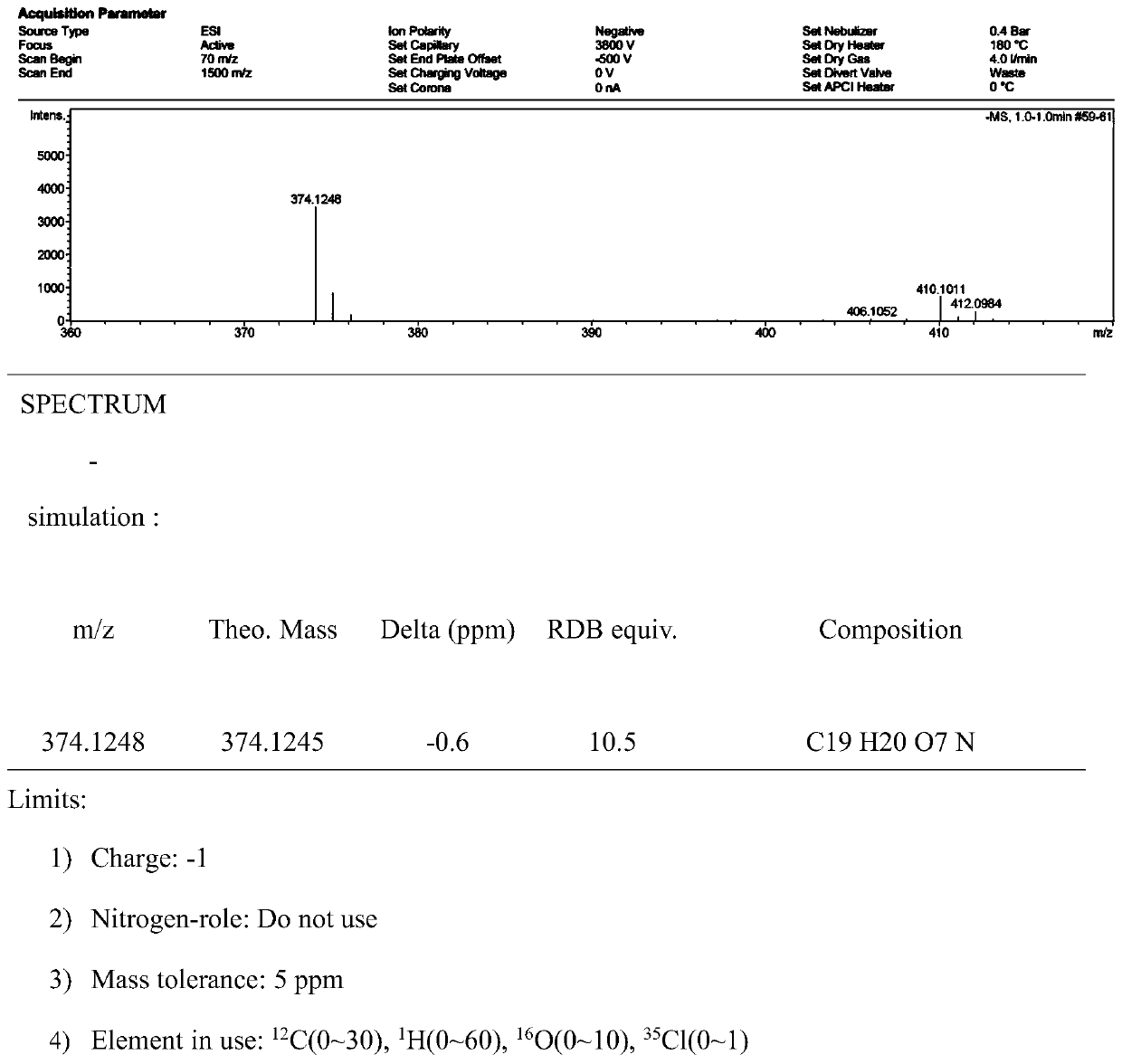
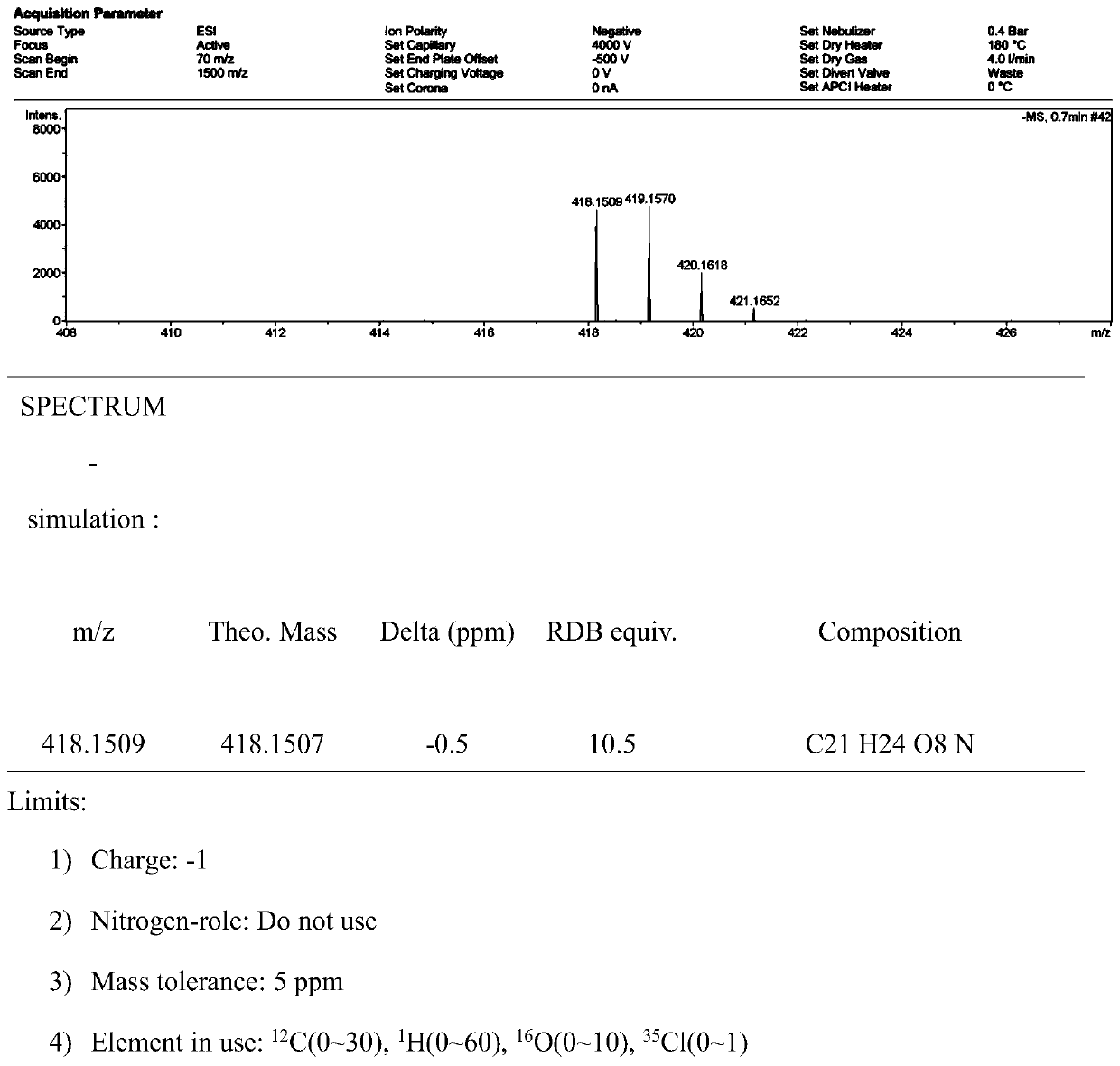
Image
Examples
Embodiment 1
[0028] A method for preparing usnic acid derivatives derived from deep-sea fungi. The usnnic acid derivatives are isolated from the fermented extract of Ochroconis sp. FS449 derived from deep-sea. The deep-sea fungus Ochroconis sp.FS449 is isolated from Indian Ocean deep-sea sediments.
[0029] The deep-sea fungus Ochroconis sp. FS449 was deposited in the Guangdong Provincial Microbial Culture Collection Center (GDMCC) on October 29, 2019, with a preservation number of GDMCC: 60825 and a classification name of Ochroconis sp. The address of the preservation unit is: 5th Floor, Building 59, Compound, No. 100 Xianlie Middle Road, Guangzhou City.
[0030] The concrete preparation method of described usnic acid derivative is as follows:
[0031] 1) Seed solution culture of the deep-sea fungus Ochroconis sp.FS449: insert the deep-sea fungus Ochroconissp.FS449 into the seed medium, and cultivate it in a shaker at a rotation speed of 120rpm at 28°C for 5 days to obtain a seed culture...
Embodiment 2
[0049] Compound 1-4 inhibitory activity test experiment of acetylcholinesterase.
[0050] Use thioacetylcholine and DTNB (5,5'-dithiobis(2-nitrobenzoic acid)) as substrates to prepare thioacetylcholine standard products in PBS, prepare 1mM compound 1-4 standard solutions with DMSO, and prepare 1mM compound 1-4 standard solutions with DMSO Dilute to different concentrations of 0.1mM, 0.05mM, 0.01mM, prepare 15mM thioacetylcholine and 15mM DTNB solutions with PBS and a small amount of EDTA, respectively. 10 μL of compounds at different concentrations were added to a mixture of 0.8 μL of acetylcholinesterase (1.0 U / mL) dissolved in PBS and 10 μL of 15 mM DTNB, and incubated at 37° C. for 2 minutes. Add 10 μL of 15 mM thioacetylcholine, incubate at 37°C for 20 minutes, add 1% SDS to terminate the reaction, measure the absorbance at 410 nm with a microplate reader (Thermo Scientific, Multiskan GO), and calculate the inhibition rate. 1 mM neostigmine methosulfate (Shanghai McLean B...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com