Arginine-based method for preventing aggregation of omalizumab
A technology of omalizumab and arginine, used in chemical instruments and methods, antibodies, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment one, with reference to Figure 1~2 , an arginine-based anti-aggregation method for omalizumab, comprising the steps of:
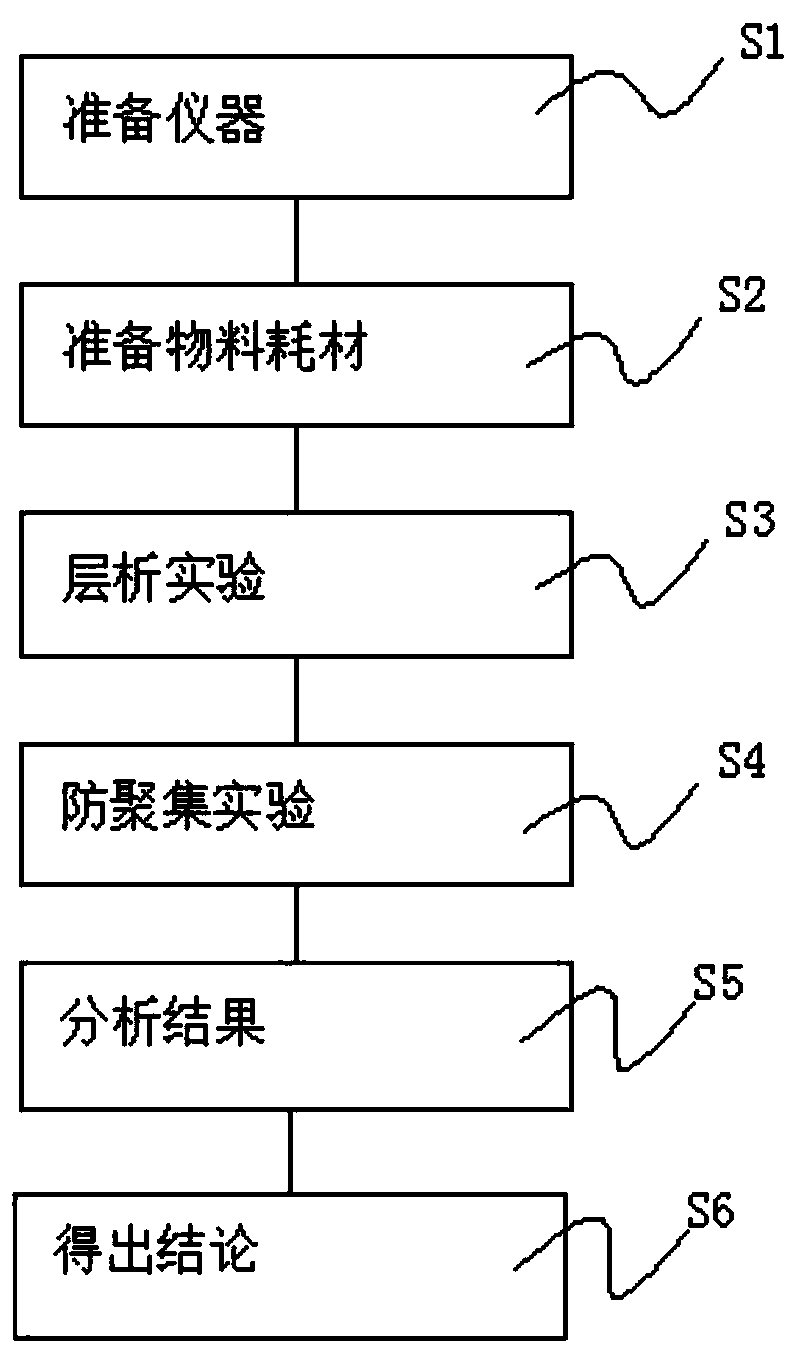
[0065] S1. Prepare instruments, prepare electronic balance, pH meter, chromatography system, pipette gun, rProtein A filler, XK50 / 20 chromatography column (As: 1.114, HETP: 0.0213, CV: 175, H: 8.9) before the experiment;
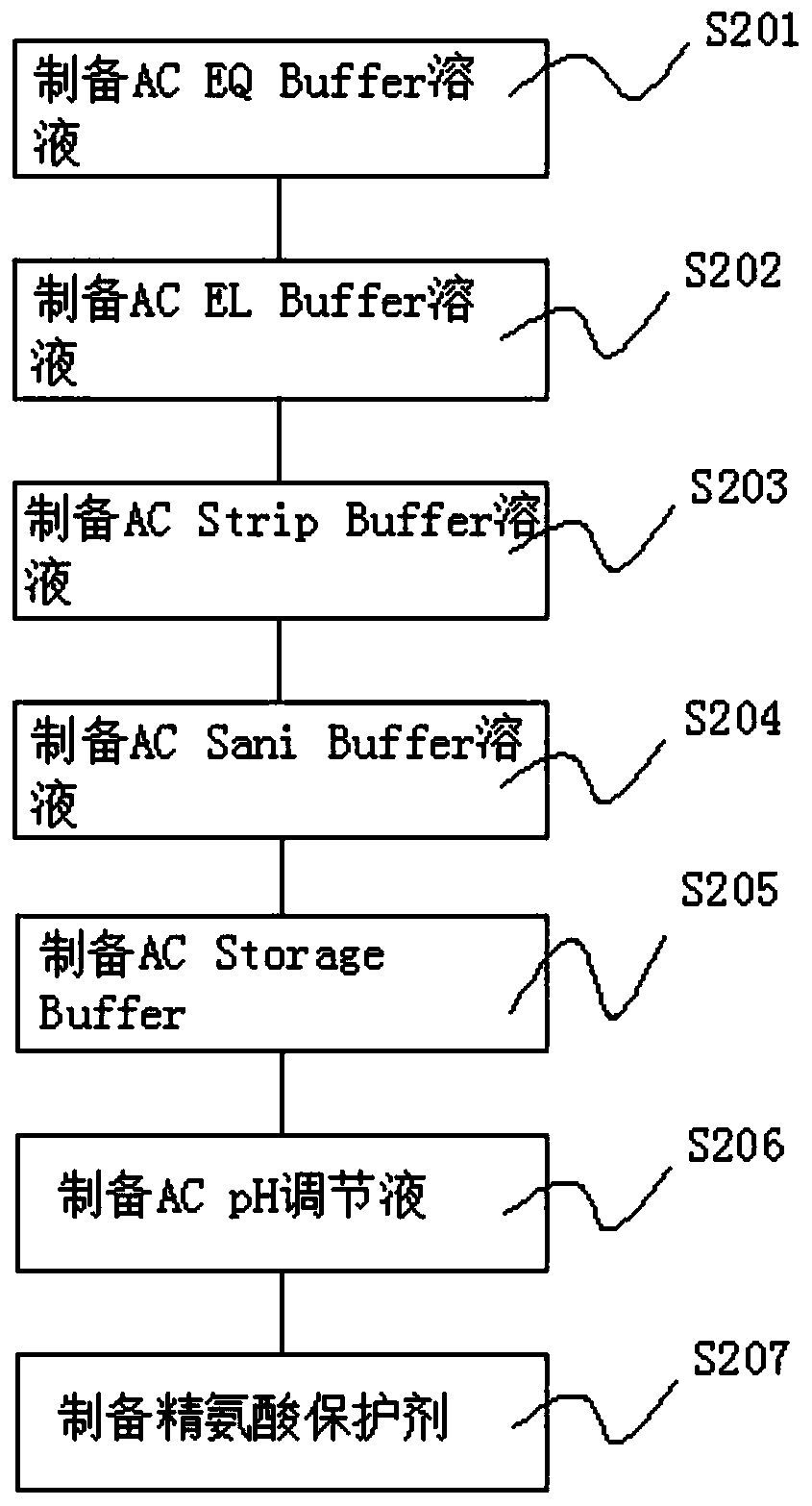
[0066] S2. Prepare materials and consumables, and prepare materials to prepare solutions for experiments;
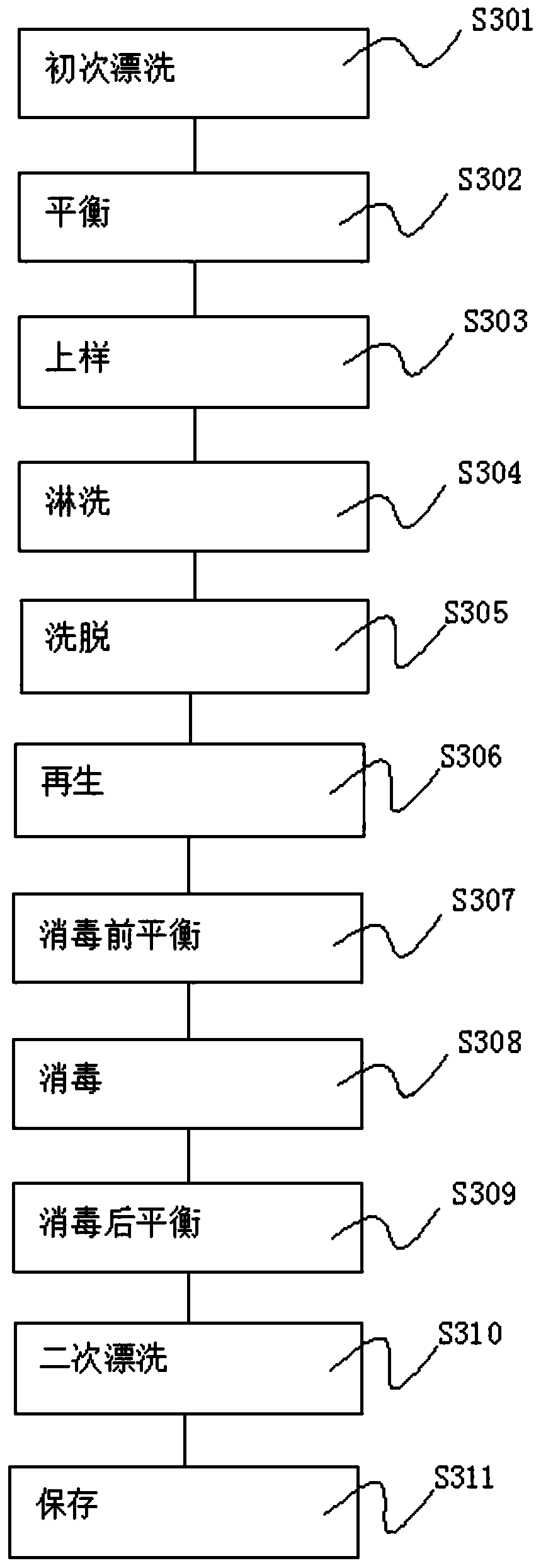
[0067] S3. Chromatography experiment, the chromatography experiment is carried out through the steps of rinsing, equilibration, sample loading, rinsing, elution, regeneration, pre-disinfection equilibration, disinfection, post-disinfection equilibration, secondary rinsing, and preservation;
[0068] S4, anti-aggregation experiment, the pH is adjusted by dilute hydrochloric acid, and Tris is added dropwise after centrifugation for observation;
[0069] S5, analyzing the results, analyzing the results under differe...
Embodiment 2
[0079] Embodiment two, refer to Figure 1~3 , an arginine-based anti-aggregation method for omalizumab, comprising the steps of:
[0080] S1. Prepare instruments, prepare electronic balance, pH meter, chromatography system, pipette gun, rProtein A filler, XK50 / 20 chromatography column (As: 1.114, HETP: 0.0213, CV: 175, H: 8.9) before the experiment;
[0081] S2. Prepare materials and consumables, and prepare materials to prepare solutions for experiments;
[0082] S3. Chromatography experiment, the chromatography experiment is carried out through the steps of rinsing, equilibration, sample loading, rinsing, elution, regeneration, pre-disinfection equilibration, disinfection, post-disinfection equilibration, secondary rinsing, and preservation;
[0083] S4, anti-aggregation experiment, the pH is adjusted by dilute hydrochloric acid, and Tris is added dropwise after centrifugation for observation;
[0084] S5, analyzing the results, analyzing the results under different conten...
Embodiment 3
[0106] Embodiment three, refer to Figure 1~4 , an arginine-based anti-aggregation method for omalizumab, comprising the steps of:
[0107] S1. Prepare instruments, prepare electronic balance, pH meter, chromatography system, pipette gun, rProtein A filler, XK50 / 20 chromatography column (As: 1.114, HETP: 0.0213, CV: 175, H: 8.9) before the experiment;
[0108] S2. Prepare materials and consumables, and prepare materials to prepare solutions for experiments;
[0109] S3. Chromatography experiment, the chromatography experiment is carried out through the steps of rinsing, equilibration, sample loading, rinsing, elution, regeneration, pre-disinfection equilibration, disinfection, post-disinfection equilibration, secondary rinsing, and preservation;
[0110] S4, anti-aggregation experiment, the pH is adjusted by dilute hydrochloric acid, and Tris is added dropwise after centrifugation for observation;
[0111] S5, analyzing the results, analyzing the results under different cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com