Hyperstable nano-drug carrier mPEG-PGlu(D)-VE(D) and preparation method and application thereof
A nano-drug carrier and a stable technology, applied in the field of ultra-stable nano-drug carriers, can solve the problems of micellar stability differences and achieve the effects of improved stability, excellent anti-oxidation and biological activity, and favorable hydrophobicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Ultra-stable nano drug carrier mPEG-PGlu(D)-VE(D), represented by formula I:
[0054]
[0055] where m=227, n=25;
[0056] The mPEG-PGlu(D)-VE(D) is an abbreviation of aminated polyethylene glycol monomethyl ether-D-polyglutamic acid-D-vitamin E.
[0057] m can also be any one of 113-273, n can also be any one of 5-50.
Embodiment 2
[0059] The preparation method of ultra-stable nano drug carrier mPEG-PGlu(D)-VE(D) (m=227, n=25), comprises the steps:
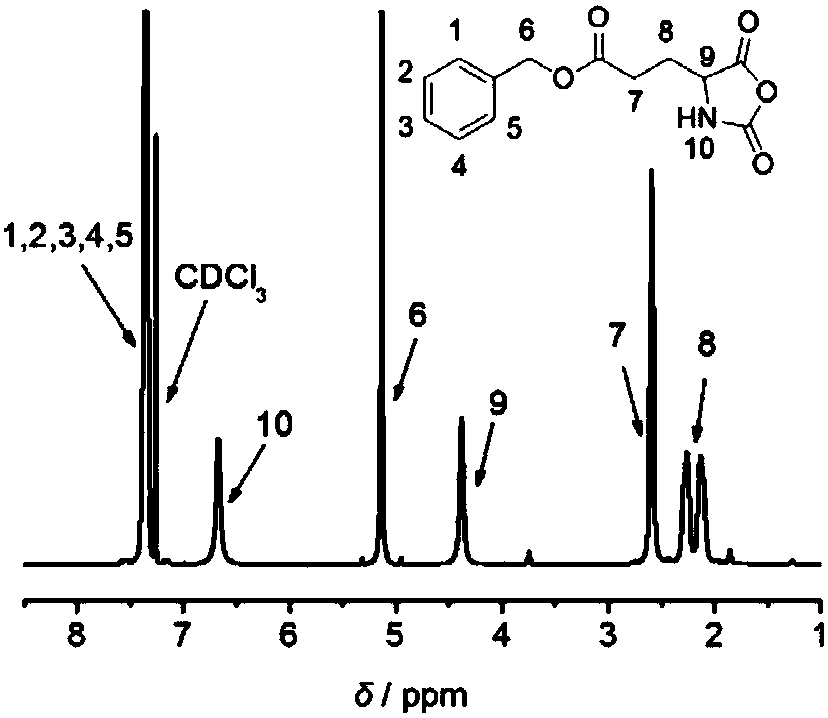
[0060] (1) Dissolve 0.5g of D-glutamic acid-5 benzyl ester (BDG) (II) and 0.31g of triphosgene in anhydrous tetrahydrofuran, react at 55°C for 90min, after the reaction, cool down to room temperature, and concentrate the solution by rotary evaporation to 2 mL, the solution was dropped dropwise into 10 mL of ice n-hexane under a low-temperature stirring reaction bath at -20°C, and a white precipitate was formed. The white precipitate obtained by suction filtration was placed in a 50mL beaker, heated and dissolved with 2mL tetrahydrofuran, and n-hexane was added dropwise until it became cloudy and did not disappear. . Suction filtration obtains and obtains D-benzyl glutamate-N-carboxylic acid anhydride (BDG-NCA) (III); see figure 1 ;
[0061] Experiments have shown that 1,4-dioxane, chloroform or ethyl acetate is used to replace the anhydrous tetrahydrofura...
Embodiment 3
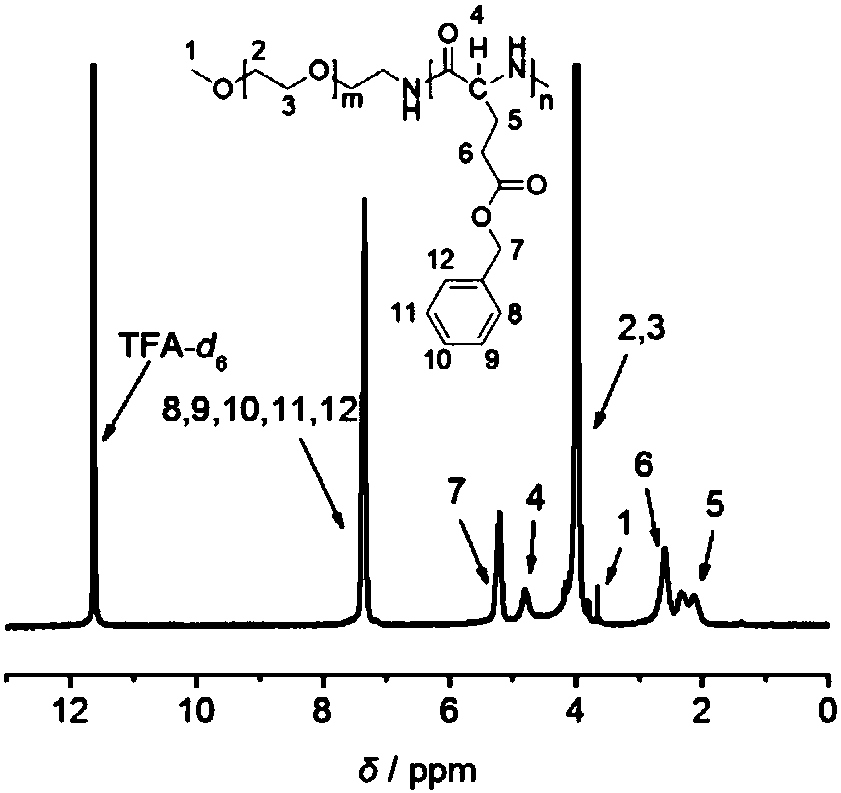
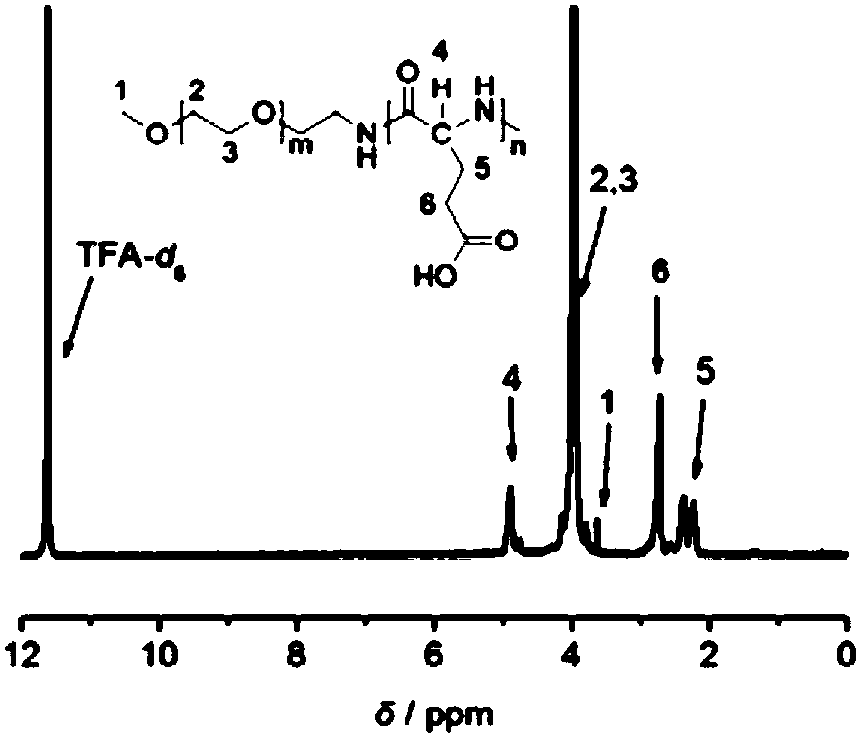
[0076] Ultra-stable nano drug carrier mPEG-PGlu(D)-VE(D) (prepared in Example 2, where m=227, n=25) and its reference substances mPEG-PGlu(D)-VE(DL), mPEG-PGlu Structural verification of (L)-VE(D), mPEG-PGlu(L)-VE(DL), mPEG-PGlu(DL)-VE(D) and mPEG-PGlu(DL)-VE(DL). (the preparation of above-mentioned reference substance is prepared with reference to the method for embodiment 2)
[0077] Prepare the concentration of 12.5μg / mL D-α-tocopherol and DL-α-tocopherol, 0.2mg / mL three kinds of polypeptide main chain mPEG-PGlu(D), mPEG-PGlu(L) and mPEG- Methanol solution of PGlu(DL) and 0.25mg / mLmPEG-PGlu(D)-VE(D) and the above five different chiral structure combinations and polypeptide vitamin E conjugate reference substance without chiral structure. Scan the UV wavelength of each sample solution;
[0078] See Figure 5 ;
[0079] In the figure, the maximum ultraviolet absorption peaks of the two vitamin Es are at 292nm, and the maximum ultraviolet absorption peaks of the three vita...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com