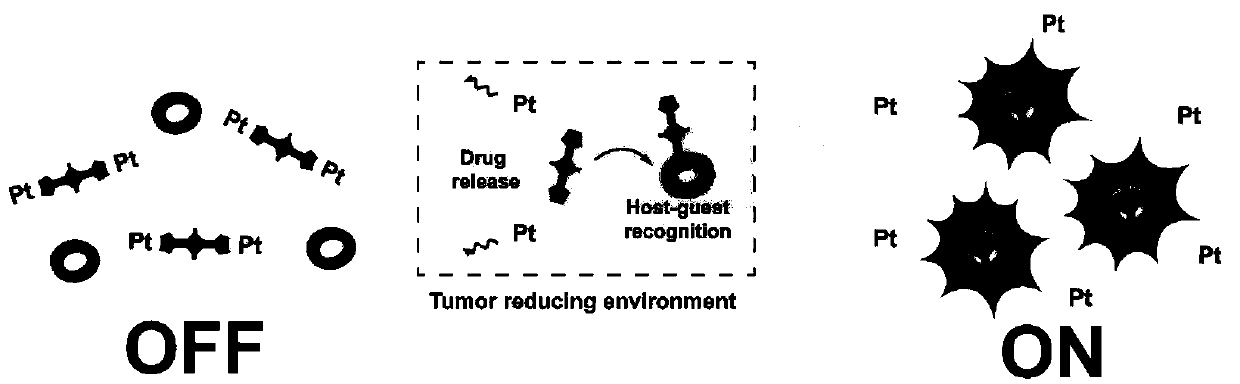
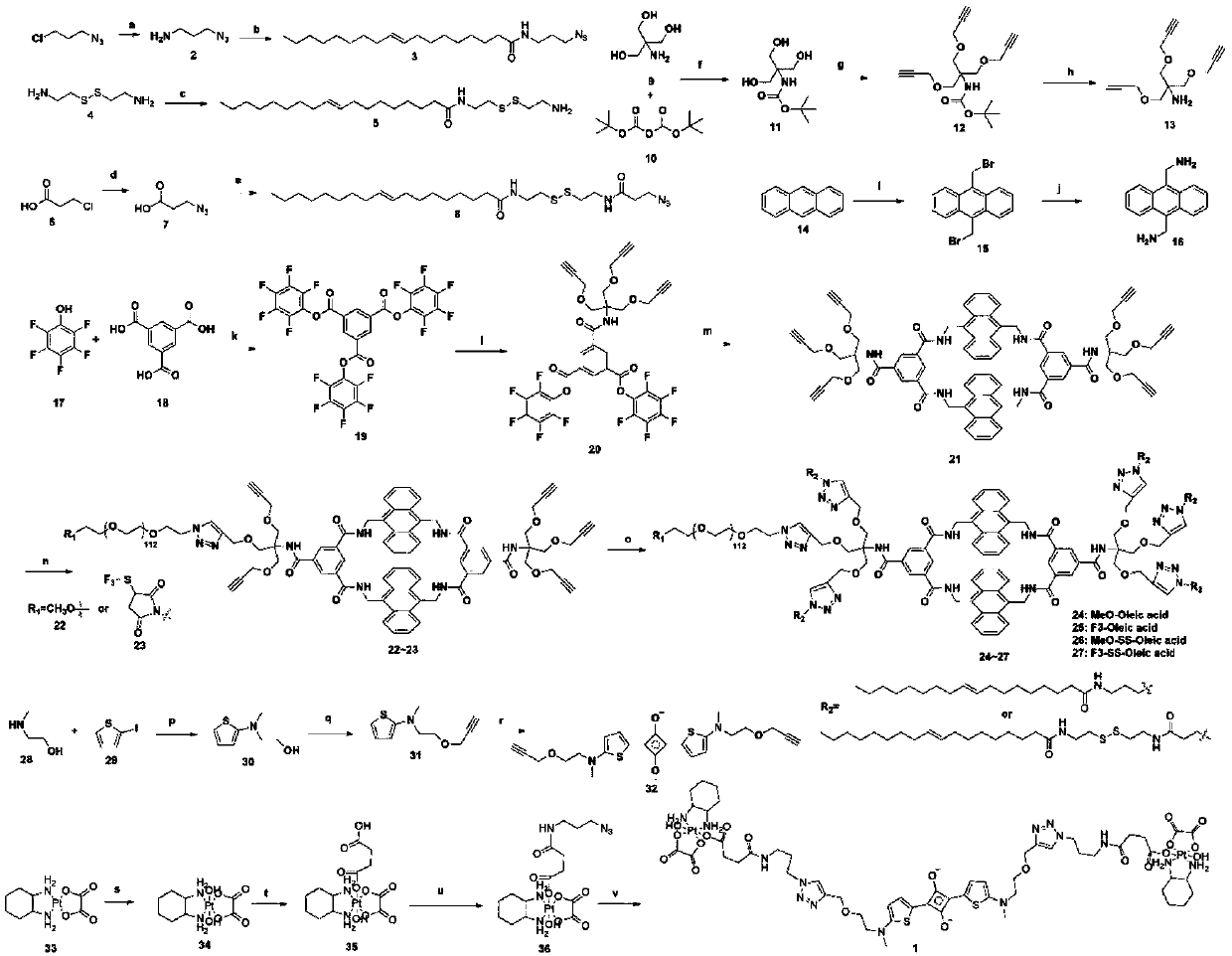
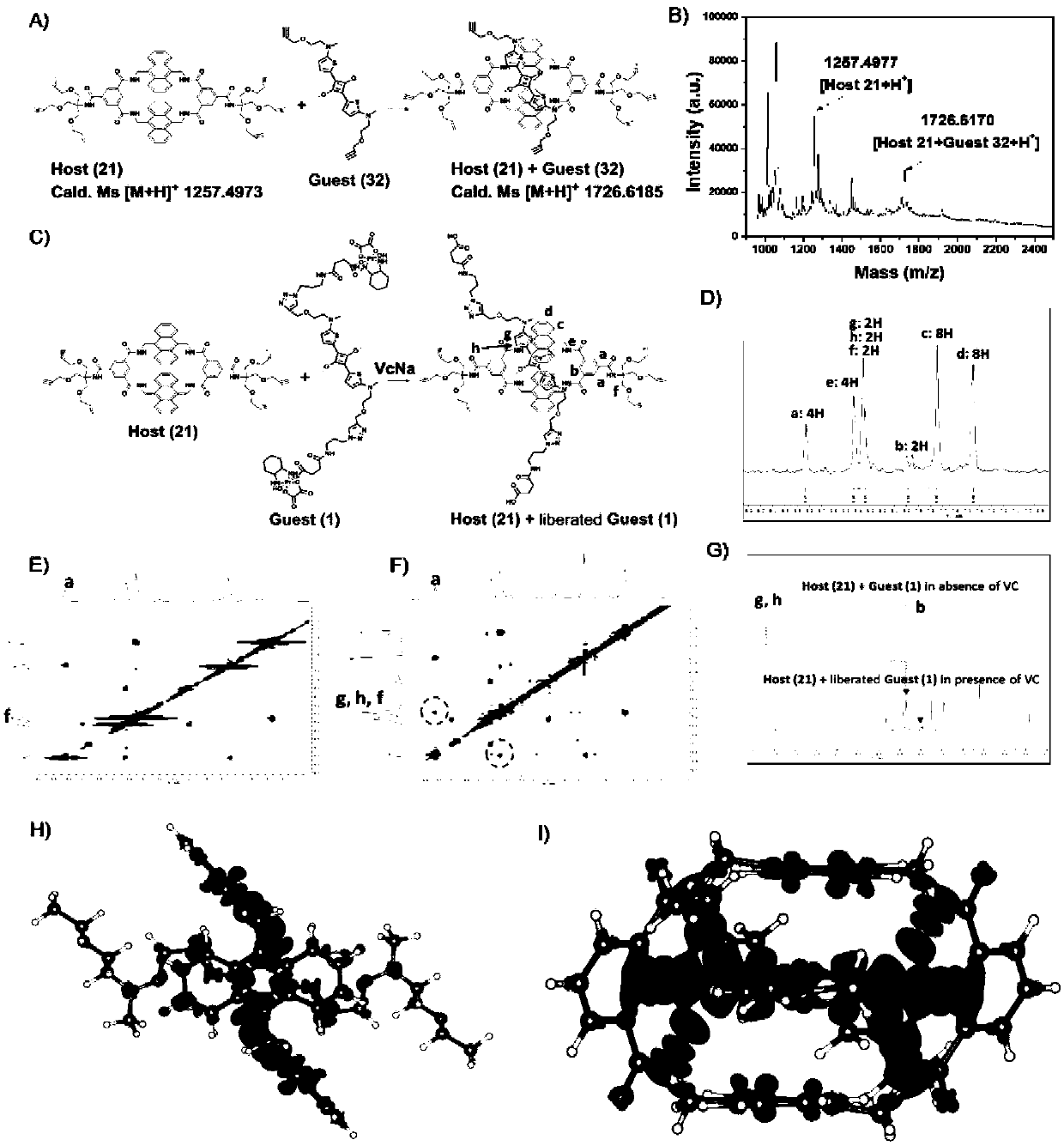
Novel diagnosis and treatment type nano-drug based on molecular shuttle
A nano-drug and molecular shuttle technology, applied in the biological field, can solve demanding problems and achieve the effect of improving curative effect and reducing systemic toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102]
[0103] 3-Chloropropylamine hydrochloride (1.00g, 7.69mmol) was dissolved in water (4mL), sodium azide (1.49g, 22.7mmol) was added, heated to 80°C and kept for 15 hours. Cool down to room temperature, add solid potassium hydroxide (1.10 g, 19.2 mmol), and extract with ether (5 mL×3). The organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain compound 2 as a colorless oily liquid (0.77 g, 100%).
[0104] Rf=0.4(dichloromethane:methanol=30:1, v:v).
[0105] 1 H NMR (400MHz, CDCl 3 ,δ,ppm):3.38(t,J=3.6Hz,2H,H-5,6),2.81(t,J=6.8Hz,2H,H-1,2),1.74(q,J=6.8Hz ,2H,H-3,4),1.75-1.00(s,w,2H,H-7,8).
[0106] FT-IR(v cm-1 ):2099.6 (vs, v N3 ),1599.6(s,δ NH2 ),1304.4(s,v C-N )...
Embodiment 2
[0108]
[0109] Oleic acid (140 mg, 0.5 mmol, 1 eq) was dissolved in dry dichloromethane (5 mL), and triethylamine (166 μL, 120 mg, 1.2 mmol, 2.4 eq) and benzo Triazole-N,N,N',N'-tetramethyluronium hexafluorophosphate (HBTU, 208mg, 0.55mmol, 1.1eq), and stirred at room temperature for half an hour. Compound 2 (250 mg, 2.5 mmol, 5 eq) was dissolved in dry dichloromethane (5 mL), and slowly added dropwise to the above solution and stirred at room temperature for four hours. The solvent was evaporated under reduced pressure, water (10 mL) was added and the pH was adjusted to 2. The above aqueous solution was extracted with petroleum ether (5 mL×3), the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain compound 3 as a wax (144 g, 79%).
[0110] Rf=0.8 (dichloromethane:ethyl acetate=30:1, v:v).
[0111] 1 H NMR (400MHz, CDCl 3 ,δ,ppm):5.60(s,w,1H,H-34),5.34(s,2H,H-18,19),3.35(t,J=6.8Hz,4H,H-35,3...
Embodiment 3
[0114]
[0115] Cystamine dihydrochloride (4,225 mg, 1 mmol, 1 eq) and triethylamine (220 mg, 2 mmol, 2 eq) were dissolved in dry tetrahydrofuran (5 mL) and stirred at room temperature for ten minutes. Oleic acid (282mg, 1mmol, 1eq), triethylamine (122mg, 1.2mmol, 1.2eq) and HBTU (400mg, 1.1eq, 1.1mmol) were dissolved in dry tetrahydrofuran (10mL) and slowly dropped into The above solution was stirred overnight at room temperature in the dark. The solvent was distilled off under reduced pressure, a saturated solution of ammonium chloride (10 mL) was added and extracted with ethyl acetate (10 mL×3). The organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain compound 5 as a yellow wax (144 g, 79%).
[0116] Rf=0.7 (dichloromethane:methanol=30:1, v:v).
[0117] 1 H NMR (400MHz, CDCl 3 ,δ,ppm):6.30(s,w,1H,H-34),5.30(s,2H,H-18,19),3.59-3.53(m,2H,H-35,36),2.81-2.73 (m,6H,H37~42),2.41-2.24(m,2H,H-43,4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| encapsulation rate | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com