Amphiphilic stellate camptothecin polymer prodrug and preparation method thereof
A technology of camptothecin and polymer, which is applied in the field of amphiphilic star camptothecin polymer prodrug and its preparation, can solve the problems of normal cell and tissue damage, drug release in advance, etc., to improve drug loading, High drug loading capacity, solving water solubility and combining effects of nano drug delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
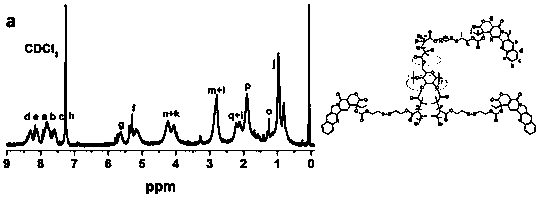
[0037] In the embodiment of the present invention, the steps of preparing the reduction-sensitive CD-PCPT-POEGMA amphiphilic star camptothecin polymer prodrug are as follows:
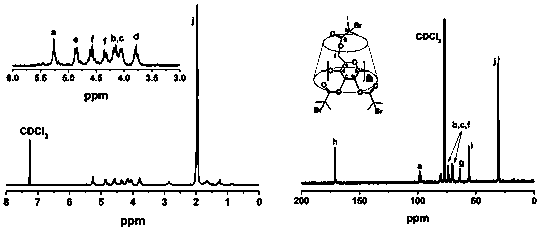
[0038] (1) Preparation of CD-Br: Under argon (Ar) atmosphere, first dissolve cyclodextrin in N-methylpyrrolidone and cool to 0℃, then dissolve the 2-bromo in N-methylpyrrolidone Isobutyryl bromide was added dropwise to the above cyclodextrin solution under stirring, reacted at 0°C for 2 hours and then at room temperature for 48 hours, and then purified to obtain the product.
[0039] (2) Preparation of MABHD: Under the condition of temperature ≤ 0℃ and argon (Ar) atmosphere, a certain amount of methacrylic acid chloride dissolved in tetrahydrofuran is added dropwise to bis(2-hydroxyethyl) disulfide, triethyl In amine and tetrahydrofuran solution, stir overnight, wash and purify, and column chromatography to obtain pure product. Wherein, the molar ratio of BHD, triethylamine, methacryloyl chloride or acryloy...
Embodiment 2
[0045] In the embodiment of the present invention, preparation of β-CD-PCPT that does not respond to stimulation cc -POEGMA amphiphilic star camptothecin polymer prodrug, the steps are as follows:
[0046] 1) Preparation of CD-Br: Under argon (Ar) atmosphere, first dissolve CD in N-methylpyrrolidone and cool to 0℃, then dissolve 2-bromoisobutyryl in N-methylpyrrolidone Bromine was added dropwise to the CD solution with stirring, reacted at 0°C for 2 hours and then at room temperature for 48 hours, and then purified to obtain the product.
[0047] (2) Preparation of MAHDO: Under the condition of temperature ≤ 0℃ and argon (Ar) atmosphere, methacrylic chloride dissolved in a certain amount of tetrahydrofuran is added dropwise to hexahexanediol (HDO), triethylamine, and tetrahydrofuran In the solution, stir overnight, wash and purify, and column chromatography to obtain a pure product. Wherein, the molar ratio of HDO, triethylamine, methacryloyl chloride or acryloyl chloride is 5:1...
Embodiment 3
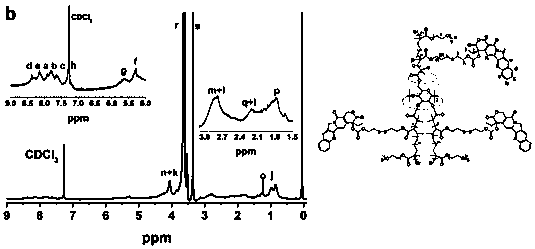
[0053] In the embodiment of the present invention, the steps of preparing the reduction-sensitive CD-PCPT-POEGMA amphiphilic star camptothecin polymer prodrug are as follows:
[0054] (1) Preparation of CD-Br: Under argon (Ar) atmosphere, first dissolve cyclodextrin in N-methylpyrrolidone and cool to 0℃, then dissolve the 2-bromo in N-methylpyrrolidone Isobutyryl bromide was added dropwise to the above cyclodextrin solution under stirring, reacted at 0°C for 2 hours and then at room temperature for 48 hours, and then purified to obtain the product.
[0055] (2) Preparation of MABHD: Under the condition of temperature ≤ 0℃ and argon (Ar) atmosphere, a certain amount of methacrylic acid chloride dissolved in tetrahydrofuran is added dropwise to bis(2-hydroxyethyl) disulfide (BHD) , Triethylamine, tetrahydrofuran solution, stir overnight, wash and purify, column chromatography to obtain a pure product. Wherein, the molar ratio of BHD, triethylamine, methacryloyl chloride or acryloyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com