Cisplatin-containing medicine, preparation method, pharmaceutical composition and application thereof
A drug, cisplatin technology, applied in the field of medicine, can solve the problems of the small molecule drug cisplatin delivery reliability, the limited clinical application of cisplatin drugs, the contradiction between toxicity and transfection activity, the difficulty of connection and non-toxic degradation, etc., to achieve The effect of high reliability, improved stability, and reduced chance of contact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0156] According to the second aspect of the present application, there is also provided a method for preparing the above-mentioned cisplatin-containing medicine, which comprises the following steps: providing any of the above-mentioned nucleic acid nanoparticles; Platinum is mounted on nucleic acid nanoparticles to obtain cisplatin-containing drugs.
[0157] When physically linked, cisplatin is usually physically intercalated between GC base pairs. When the connection is made by covalent connection, cisplatin usually chemically reacts with the amino group outside the G ring to form a covalent connection. The cisplatin-containing medicine prepared by the above-mentioned method can have better targeting ability after being modified by the target head, can deliver cisplatin stably, and has high reliability.
[0158] In a preferred embodiment, the step of mounting cisplatin by physical connection includes: mixing and stirring cisplatin, nucleic acid nanoparticles and a first sol...
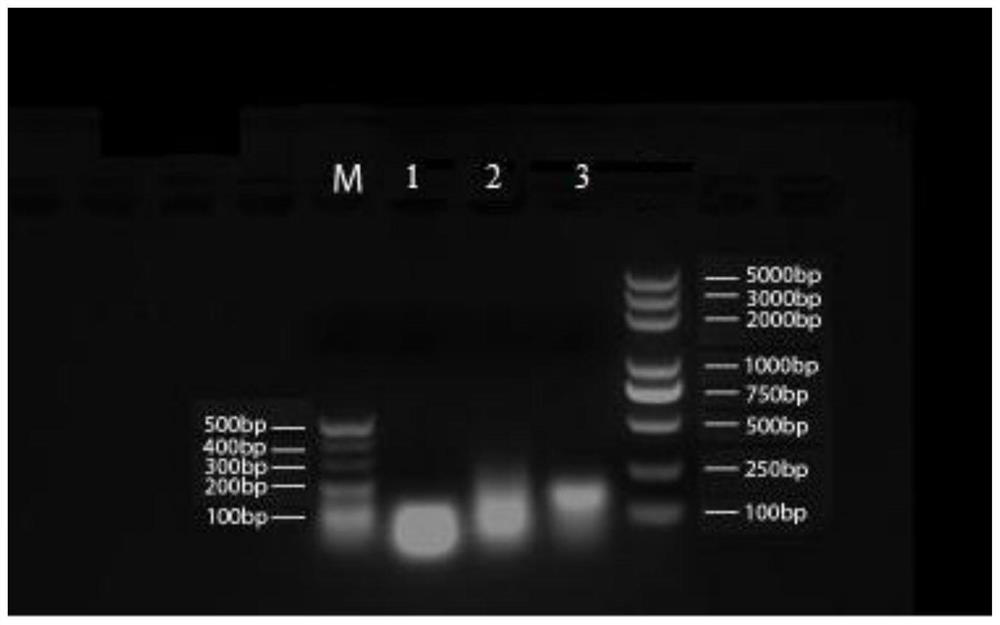
Embodiment 1
[0181] 1. RNA and DNA nanoparticle carriers:
[0182] (1) The base sequences of the three polynucleotides constituting the RNA nanoparticles are shown in Table 1:
[0183] Table 1:
[0184]
[0185]
[0186] (2) Three polynucleotide base sequences of DNA nanoparticles
[0187] The DNA adopts the same sequence as the RNA described above, except that T replaces U. Among them, the molecular weight of the a chain is 8802.66, the molecular weight of the b chain is 8280.33, and the molecular weight of the c chain is 9605.2.
[0188] The a, b and c chains of the above RNA nanoparticles and DNA nanoparticles were all synthesized by Sangon Bioengineering (Shanghai) Co., Ltd.
[0189] Second, the self-assembly experimental steps:
[0190] (1) Dissolve RNA or DNA single strands a, b, and c in DEPC water or TMS buffer at a molar ratio of 1:1:1;
[0191] (2) Heating the mixed solution to 80°C / 95°C (wherein the RNA assembly temperature is 80°C and the DNA assembly temperature is ...
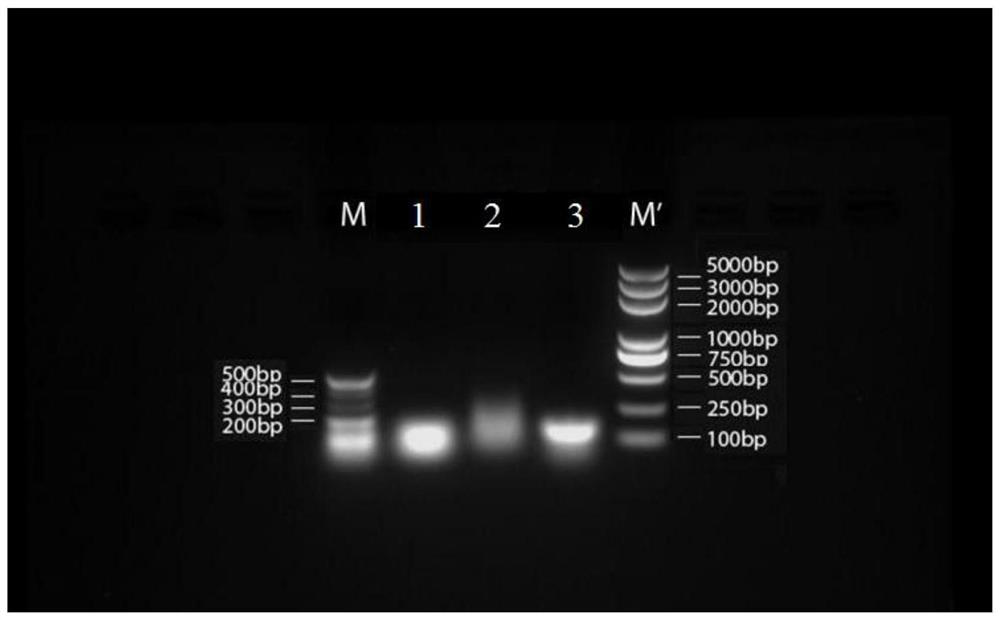
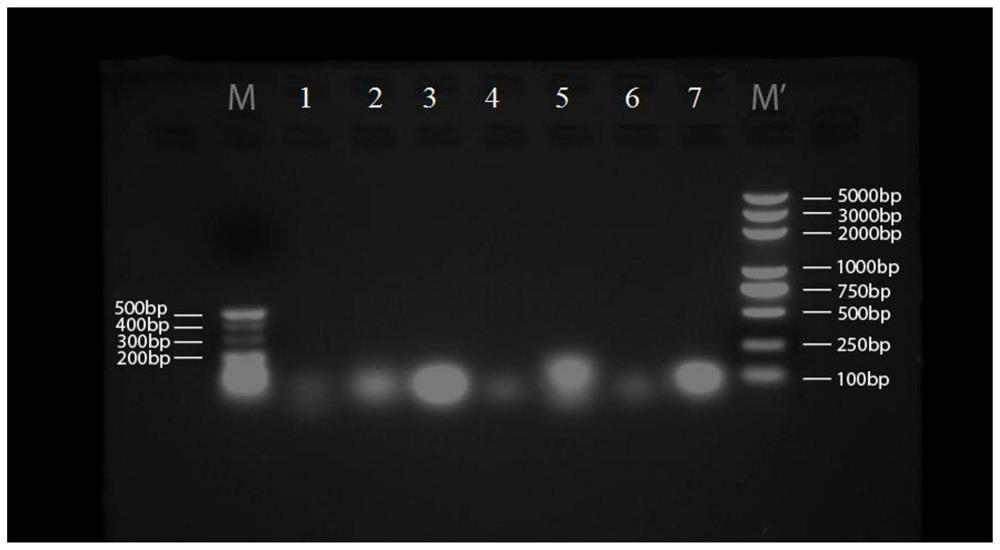
Embodiment 2
[0202] 1. 7 groups of short-sequence RNA nanoparticle carriers:
[0203] (1) The base sequences of the three polynucleotides that make up the 7 groups of RNA nanoparticles are shown in Tables 2 to 8:
[0204] Table 2: R-1
[0205]
[0206] Table 3: R-2
[0207]
[0208] Table 4: R-3
[0209]
[0210] Table 5: R-4
[0211]
[0212] Table 6: R-5
[0213]
[0214] Table 7: R-6
[0215]
[0216] Table 8: R-7
[0217]
[0218] The single strands of the above seven groups of short-sequence RNA nanoparticle carriers were all synthesized by Sangon Bioengineering (Shanghai) Co., Ltd.
[0219] Second, the self-assembly experimental steps:
[0220] (1) Mix and dissolve RNA single strands a, b, and c simultaneously in DEPC water or TMS buffer at a molar ratio of 1:1:1;
[0221] (2) heating the mixed solution to 80°C, keeping the temperature for 5min and then slowly cooling to room temperature at a rate of 2°C / min;
[0222] (3) Load the product onto an 8% (m / v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com