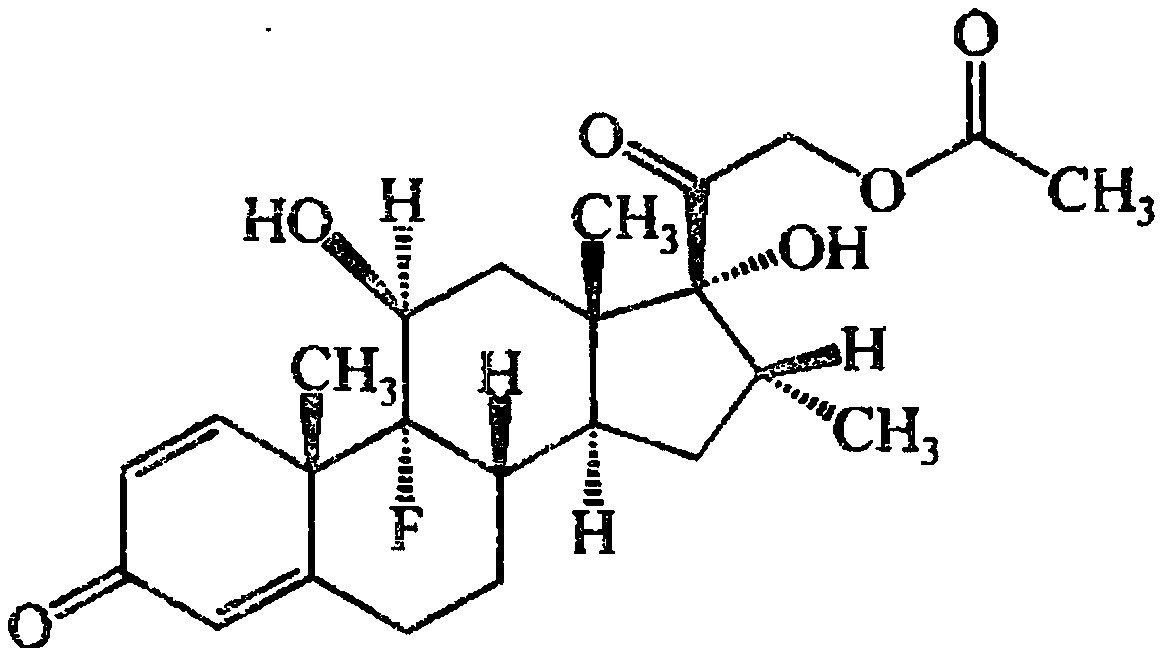
Dexamethasone acetate injection and preparation method
A technology of dexamethasone acetate and injection, which is applied in the field of medicine and can solve problems such as insufficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Embodiment 1: preparation dexamethasone acetate injection
[0097] prescription:
[0098] Dexamethasone acetate 5mg,
[0100] 1.5mg Polysorbate 80,
[0101] Thimerosal 10μg,
[0102] Carmellose Sodium 5mg,
[0103] Add water for injection to 1ml.
[0104] Preparation method:
[0105] (1) Add sodium chloride to the water for injection (the temperature is 55 ℃) of formula volume 75% to dissolve;
[0106] (2) Add thimerosal to the sodium chloride solution, stir to dissolve, then slowly add carmellose sodium and stir to disperse, continue stirring to fully dissolve, filter (pass through a 30-mesh filter screen), heat up and boil;
[0107] (3) Add polysorbate 80 and dexamethasone acetate to the above-mentioned boiled solution, continue to boil for 30 minutes to fully dissolve the medicinal solution, cool to room temperature, add water for injection to 85-90% of the formula volume;
[0108] (4) Carry out the shear cycle 45min with the me...
Embodiment 2
[0110] Embodiment 2: preparation dexamethasone acetate injection
[0111] prescription:
[0112] Dexamethasone acetate 4.5mg,
[0113] Sodium chloride 7.5mg,
[0114] 1.6mg polysorbate 80,
[0115] Thimerosal 9μg,
[0116] Carmellose Sodium 5.5mg,
[0117] Add water for injection to 1ml.
[0118] Preparation method:
[0119] (1) Add sodium chloride to the water for injection (the temperature is 50 ℃) of formula volume 80% to dissolve;
[0120] (2) Add thimerosal to the sodium chloride solution, stir to dissolve, then slowly add carmellose sodium and stir to disperse, continue stirring to fully dissolve, filter (pass through a 30-mesh filter screen), heat up and boil;
[0121] (3) Add polysorbate 80 and dexamethasone acetate to the above boiled solution, continue to boil for 35 minutes to fully dissolve the medicinal solution, cool to room temperature, add water for injection to 85-90% of the formula volume;
[0122] (4) Use a high-shear homogeneous emulsifier to shea...
Embodiment 3
[0124] Embodiment 3: preparation dexamethasone acetate injection
[0125] prescription:
[0126] Dexamethasone acetate 5.5mg,
[0127] Sodium chloride 8.5mg,
[0128] 1.4mg polysorbate 80,
[0129] Thimerosal 11μg,
[0130] Carmellose Sodium 4.5mg,
[0131] Add water for injection to 1ml.
[0132] Preparation method:
[0133] (1) Add sodium chloride to the water for injection (the temperature is 60° C.) of 70% of the formula volume to dissolve;
[0134] (2) Add thimerosal to the sodium chloride solution, stir to dissolve, then slowly add carmellose sodium and stir to disperse, continue stirring to fully dissolve, filter (pass through a 30-mesh filter screen), heat up and boil;
[0135] (3) Add polysorbate 80 and dexamethasone acetate to the above-mentioned boiled solution, continue to boil for 25 minutes to fully dissolve the medicinal solution, cool to room temperature, and add water for injection to 85-90% of the formula volume;
[0136] (4) Use a high-shear homogene...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com