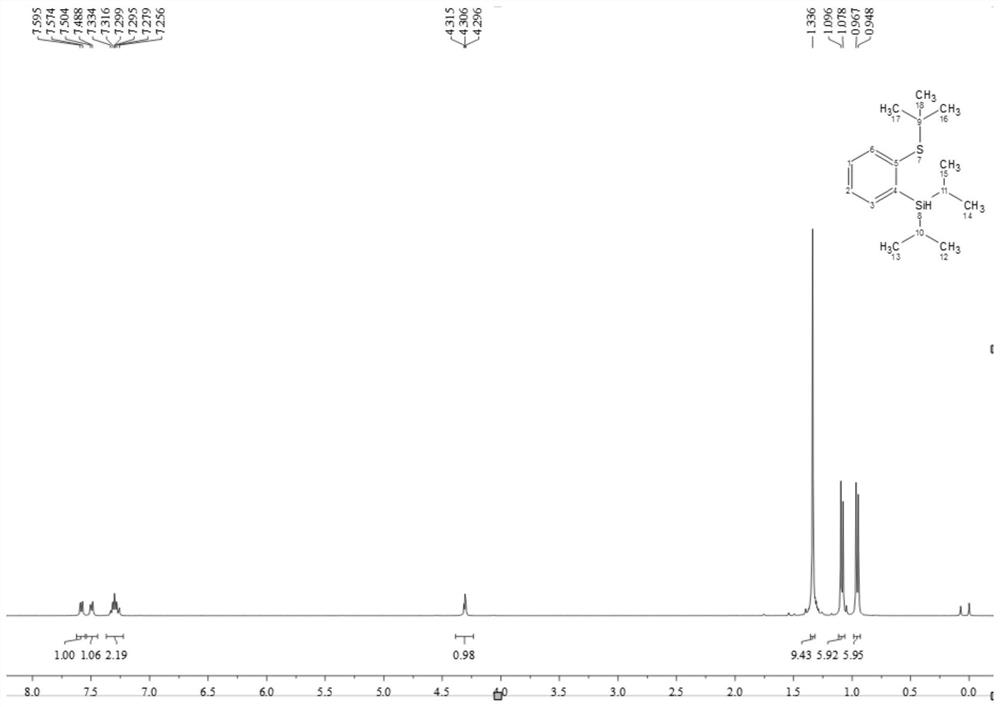
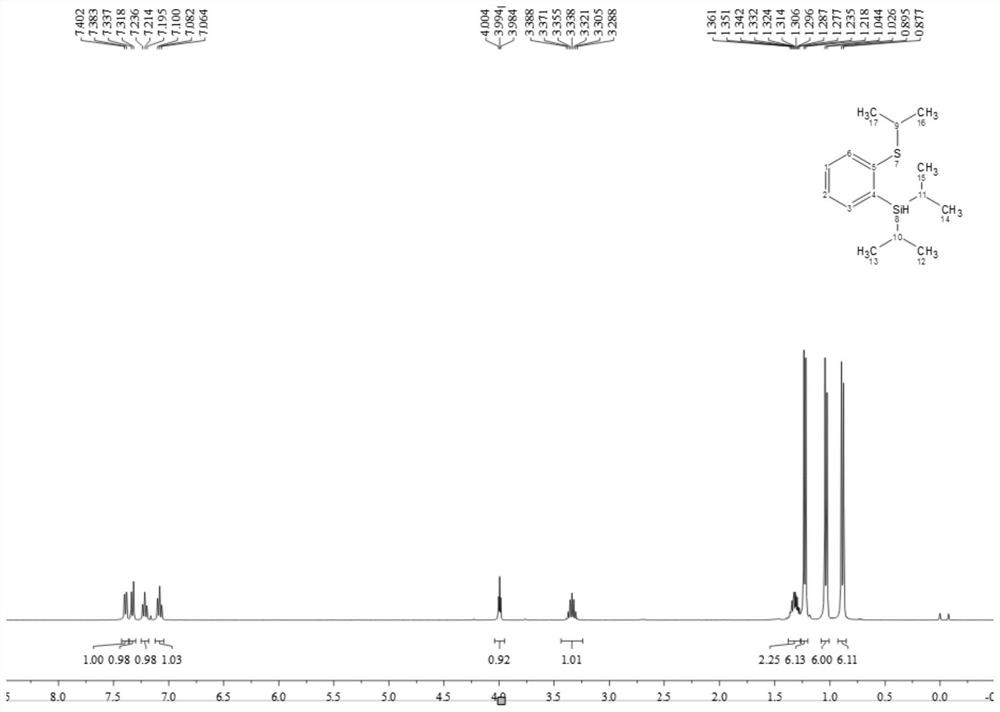
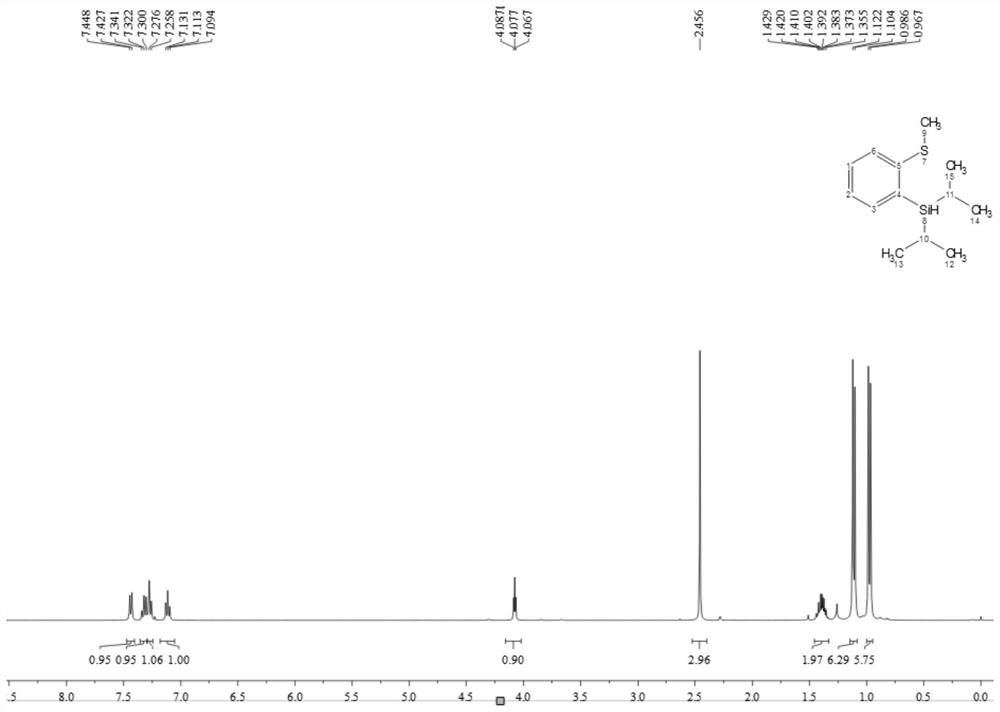
A kind of thiosilane ligand and its preparation method and its application in aryl borylation catalytic reaction
A technology of thiosilane, aryl borate, applied in physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, organic silicon compound, etc., can solve the problem of increasing separation difficulty and cost, and is not suitable Industrial production, complex synthesis and other problems, to achieve the effect of low cost of raw materials, easy separation and purification, and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The preparation method of described thiosilane ligand comprises the following steps:
[0031] Under the protection of nitrogen, the molar ratio of 2-bromophenylene sulfide compound and n-butyllithium is 1:(1.1-1.3), and react in tetrahydrofuran at -30~-80°C for 1-2 hours to obtain lithium bromide exchanged thioether compounds, and then adding diisopropylchlorosilane to the thioether compounds exchanged with lithium bromide, slowly returning to room temperature, after the reaction, quenching the reaction with sodium bicarbonate, extracting with ether, and concentrating the organic phase to obtain The crude product is separated and purified by silica gel chromatography to obtain the thiosilane ligand as a pale yellow oily liquid. The molar ratio of bromide-lithium-exchanged thioether compounds to diisopropylchlorosilane is 1:(1.2~1.5),
[0032] The 2-bromophenylene sulfide compound is a compound having a structure shown in formula L1', L2' or L3'.
[0033]
[0034] T...
specific Embodiment
[0052] (1) Preparation of thiosilane ligands
[0053] The thiosilane ligand is a compound having a structure as shown in formula L1, L2 or L3:
[0054]
[0055] Examples of preparation methods:
preparation example 1
[0057] 1) First put 20ml of water into the flask, then add 25ml of concentrated sulfuric acid, put it at zero degree, then add 2-bromothioanisole (1.89g, 10mmol) and tert-butanol (1.11mg, 15mmol) and mix and stir, the reaction starts Place at room temperature, stir for 8 hours, wait for the reaction to stop, put the reaction at zero, add 30ml×3 ether to extract the organic phase, take the organic phase and add appropriate amount of sodium bicarbonate to adjust the pH=8-10, extract with ether, filter, and dry with anhydrous sodium carbonate , and the solvent was spin-dried to obtain a crude product. The crude product was purified by column chromatography (using 300-mesh silica gel, the mass ratio of silica gel to the substance to be purified was 50:1, the eluent was petroleum ether and ethyl acetate, and the volume ratio was 50:1) to obtain a yellow oily liquid with a yield of 85 %(2.08g), identified by 1H NMR (400MHz, Chloroform-d) δ7.65(t, J=6.9Hz, 2H), 7.25(t, J=7.5Hz, 1H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com