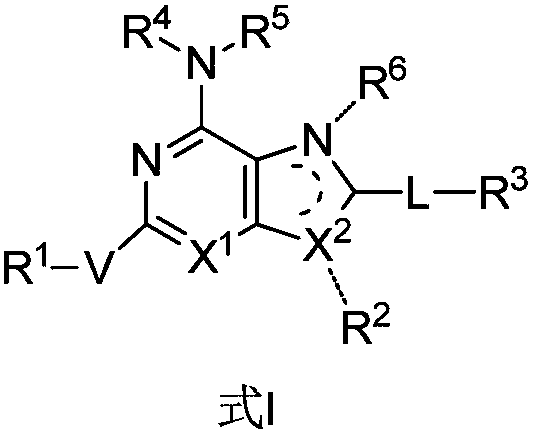
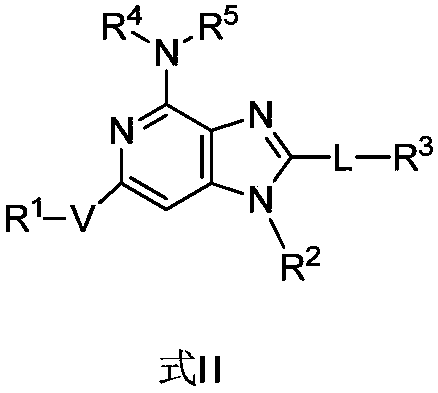
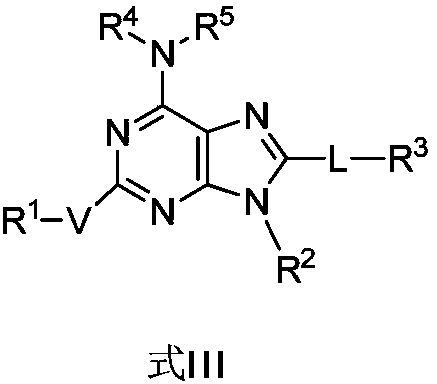
Bicyclic compound, preparation method and application thereof
A technology of compounds and mixtures used in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0357] Example 1: 3-(4-amino-1-methyl-6-(5-phenylthiophen-2-yl)-1H-imidazo[4,5-c]pyridin-2-yl)propane-1 - alcohol (1)
[0358]
[0359] The first step: 2,6-dichloro-N-methyl-3-nitropyridin-4-amine (1b)
[0360] Compound 1a (5.0g, 24.15mmol), K 2 CO 3 (6.66g, 48.30mmol) was added into 40mL of acetonitrile, heated to 80°C and stirred for 12h. After the reaction was completed, it was filtered with diatomaceous earth, and the filtrate was spin-dried and subjected to flash column chromatography (eluent system B) to obtain compound 1b (4.80 g). MS(ESI,m / z):222.0[M+H] + .
[0361] The second step: 6-chloro-N-methyl-3-nitro-N-(2,4,4-trimethylpentan-2-yl)pyridine-2,4-diamine (1c)
[0362] Compound 1b (4.80g, 21.71mmol), tert-octylamine (5.61g, 43.42mmol) and triethylamine (6.58mg, 65.13mmol) were added to 40mL of dichloromethane, heated to 40°C and stirred for 12h. After the reaction was completed, the solvent was spin-dried and subjected to flash column chromatography (eluen...
Embodiment 2
[0379] Example 2: 3(4-amino-6-(5-(3-fluorophenyl)thiophen-2-yl)-1-methyl-1H-imidazo[4,5-c]pyridin-2-yl ) propan-1-ol (2)
[0380]
[0381] The first step: 2-(3-(benzyloxy)propyl)-6-(5-(3-fluorophenyl)thiophen-2-yl)-1-methyl-1H-imidazo[4, 5-c]pyridin-4-amine (2a)
[0382] Compound 1h (32.12mg, 70.22μmol), 3-fluorophenylboronic acid (19.65mg, 140.45μmol), Pd(dppf)Cl 2 ·CH 2 Cl 2 (6mg, 7.02μmol), potassium carbonate (19.41mg, 140.45μmol) were added to 5mL 1,4-dioxane and 0.5mL water, N 2 Heated to 90°C under protection for 5h. After the reaction was completed, it was filtered with celite and purified by flash column chromatography (elution system A) to obtain compound 2a (20 mg). MS(ESI,m / z):473.2[M+H] + .
[0383] The second step: 3(4-amino-6-(5-(3-fluorophenyl)thiophen-2-yl)-1-methyl-1H-imidazo[4,5-C]pyridin-2-yl ) propan-1-ol (2)
[0384] Compound 2a (20 mg, 12.33 μmol) was dissolved in 4 mL of trifluoroacetic acid, N 2 Under protection, the reaction was heated t...
Embodiment 3
[0387] Example 3: 3-(4-amino-6-(5-(4-methoxyphenyl)thiophen-2-yl)-1-methyl-1H-imidazo[4,5-c]pyridine- 2-yl)propan-1-ol (3)
[0388]
[0389] The first step: 2-(3-(benzyloxy)propyl)-6-(5-(4-methoxyphenyl)thiophen-2-yl)-1-methyl-1H-imidazo[ 4,5-c]pyridin-4-amine (3a)
[0390] Compound 1h (90mg, 127.90μmol), 4-methoxyphenylboronic acid (38.87mg, 255.80μmol), Pd(dppf)Cl 2 ·CH 2 Cl 2 (12mg, 14.04μmol), potassium carbonate (53.03mg, 383.70μmol) were added to 5mL 1,4-dioxane and 0.5mL water, N 2 Heated to 90°C under protection for 5h. After the reaction was completed, it was filtered with celite and purified by flash column chromatography (elution system A) to obtain compound 3a (35 mg). MS(ESI,m / z):485.2[M+H] + .
[0391] The second step: 3-(4-amino-6-(5-(4-methoxyphenyl)thiophen-2-yl)-1-methyl-1H-imidazo[4,5-c]pyridine- 2-yl)propan-1-ol (3)
[0392] Compound 3a (35 mg, 72.3 μmol) was dissolved in 4 mL of trifluoroacetic acid, N 2 Under protection, the reaction was hea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com