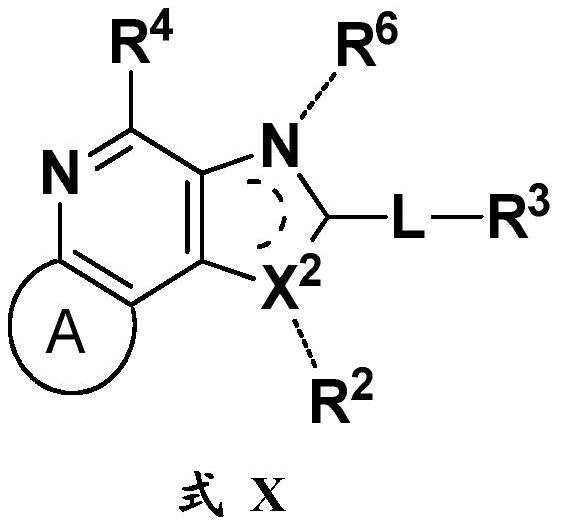
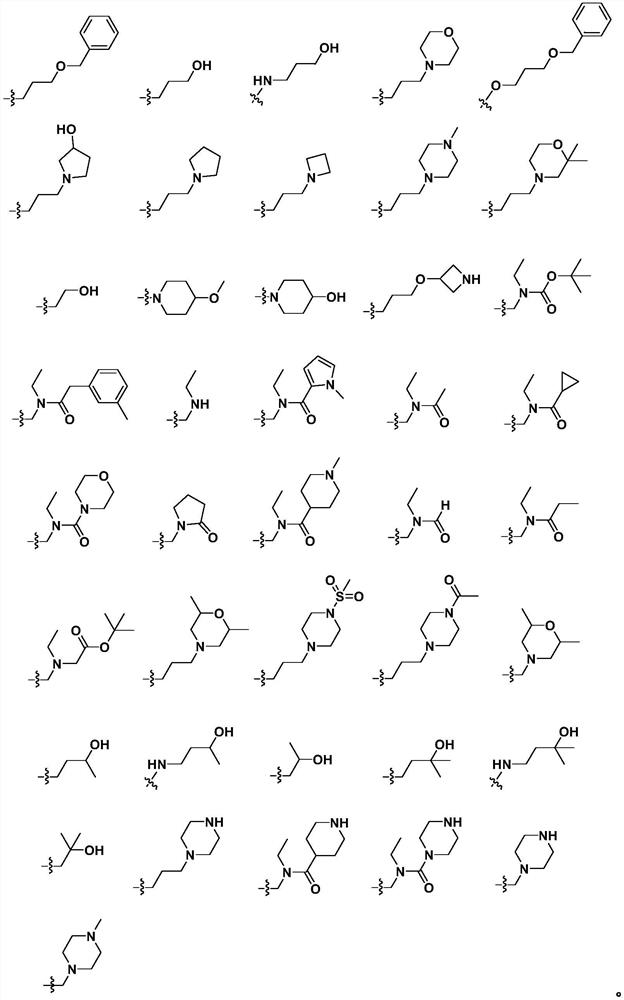
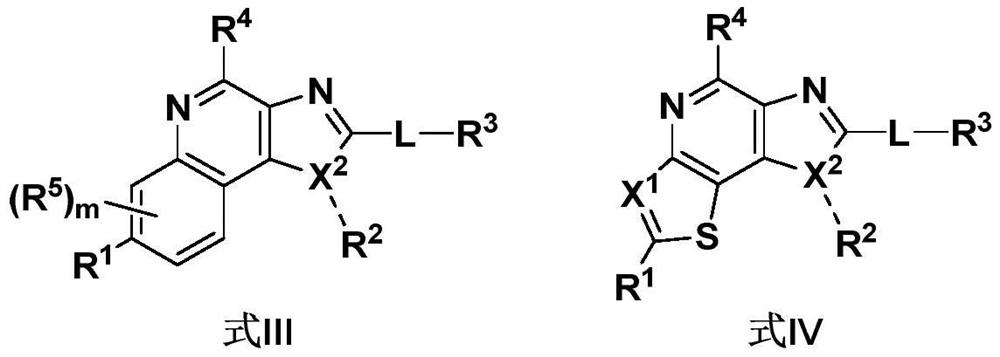
Fused ring compound, and preparation method and application thereof
A technology of compounds and mixtures, applied in the field of new fused-ring compounds, can solve the problems of colorectal tumor and colorectal cancer liver metastases and deterioration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0238] Example 1: 2-(3-(Benzyloxy)propyl)-1,4-dimethyl-7-(1H-pyrazol-3-yl)-1H-imidazo[4,5-d]thiophene And[3,2-b]pyridine(1)
[0239] 3-(1,4-Dimethyl-7-(1H-pyrazol-3-yl)-1H-imidazo[4,5-d]thieno[3,2-b]pyridin-2-yl)propane Alcohol (2)
[0240]
[0241] The first step: the synthesis of compound (4-benzyloxy)-(7-(methylamino)thieno[3,2-b]pyridyl-6-yl)butyramide (1b)
[0242] DIPEA (1.44g, 11.16mmol), HATU (1.06g, 2.79mmol) were sequentially added to 4-benzyloxybutanoic acid (541.80mg, 2.79mmol), compound 1a (500mg, 2.79mmol) in DMF (4mL) , and then kept stirring at room temperature for 5hr. The reaction was quenched with water and extracted with dichloromethane. The organic layer was dried and concentrated to obtain compound 1b (850 mg).
[0243] MS(ESI,m / z):356.1[M+H] +
[0244] The second step: the synthesis of compound 2-(3-(benzyloxy)propyl)-1-methyl-1H-imidazo[4,5-d]thieno[3,2-b]pyridine (1c)
[0245]Sodium hydroxide (184.55 mg, 4.61 mmol) was added to compound 1b (...
Embodiment 2
[0261] Example 2: 1,4-Dimethyl-2-(3-(4-methylpiperazin-1-yl)propyl)-7-(1H-pyrazol-3-yl)-1H-imidazo [4,5-d]thieno[3,2-b]pyridine (3)
[0262] 1,4-Dimethyl-2-(3-(4-methylpiperazin-1-yl)propyl)-7-(1H-pyrazol-3-yl)-1H-imidazo[4,5 -d]thieno[3,2-b]pyridine hydrochloride (3a)
[0263]
[0264] The first step: 3-(7-bromo-1,4-dimethyl-1H-imidazo[4,5-d]thieno[3,2-b]pyridin-2-yl)propyl-1- Alcohol (2a)
[0265] Compound 1f (320mg, 0.74mmol) was added into 3mL of trifluoroacetic acid, heated to 80°C and stirred for 16h. The reaction solution was concentrated, redissolved in 3 mL of MeOH, adjusted to pH=9.0 with 2N sodium hydroxide solution, and continued to stir for 30 min. Compound 2a (180 mg) was obtained by flash column chromatography (eluent system A). MS(ESI,m / z):340.0[M+H] + .
[0266] The second step: 7-bromo-2-(3-chloropropane)-1,4-dimethyl-1H-imidazo[4,5-d]thieno[3,2-b]pyridine (2b)
[0267] Compound 2a (180mg, 0.53mmol), DMF (4mg, 0.05mmol) was added to 4mL thionyl chl...
Embodiment 3
[0276] Example 3: 2-(3-(azetidin-1-yl)propyl)-1,4-dimethyl-7-(1H-pyrazol-3-yl)-1H-imidazo[ 4,5-d]thieno[3,2-b]pyridine (5)
[0277]
[0278] The first step: 2-(3-(azetidin-1-yl)propyl)-7-bromo-1,4-dimethyl-1H-imidazo[4,5-d]thieno[ 3,2-b]pyridine (3a)
[0279]Compound 2b (50mg, 0.14mmol), azetidine (48mg, 0.84mmol), tetrabutylammonium iodide (10mg, 0.03mmol), TEA (85mg, 0.84mmol) were added in 3mL of toluene, N 2 Heated to 100°C under protection for 16h. The reaction solvent was spin-dried to obtain compound 3a (53 mg), and the crude product was directly used in the next step. MS(ESI,m / z):379.1[M+H] + .
[0280] The second step: 2-(3-(azetidin-1-yl)propyl)-1,4-dimethyl-7-(1H-pyrazol-3-yl)-1H-imidazo[ 4,5-d]thieno[3,2-b]pyridine (5)
[0281] Compound 3a (53mg, 0.13mmol), 1H-pyrazole-3-boronic acid pinacid (61mg, 0.31mmol), Pd(dppf)Cl 2 (10mg, 0.01mmol), sodium carbonate (40mg, 0.38mmol) was added to the mixed solvent of 4mL DMF and 1mL water, N 2 Heated to 100°C unde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com