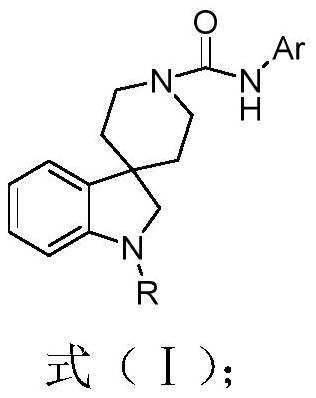
Indoline piperidine urea TRPV1 antagonistic and MOR agonistic double-target drug as well as preparation method and application thereof
An indoline piperidinyl urea, dual-target technology, applied in the directions of drug combinations, pharmaceutical formulations, antipyretics, etc., can solve the problems of reducing analgesic effect, drug tolerance and increasing drug dosage, and reduce side effects. , the effect of broad analgesic application prospects and practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
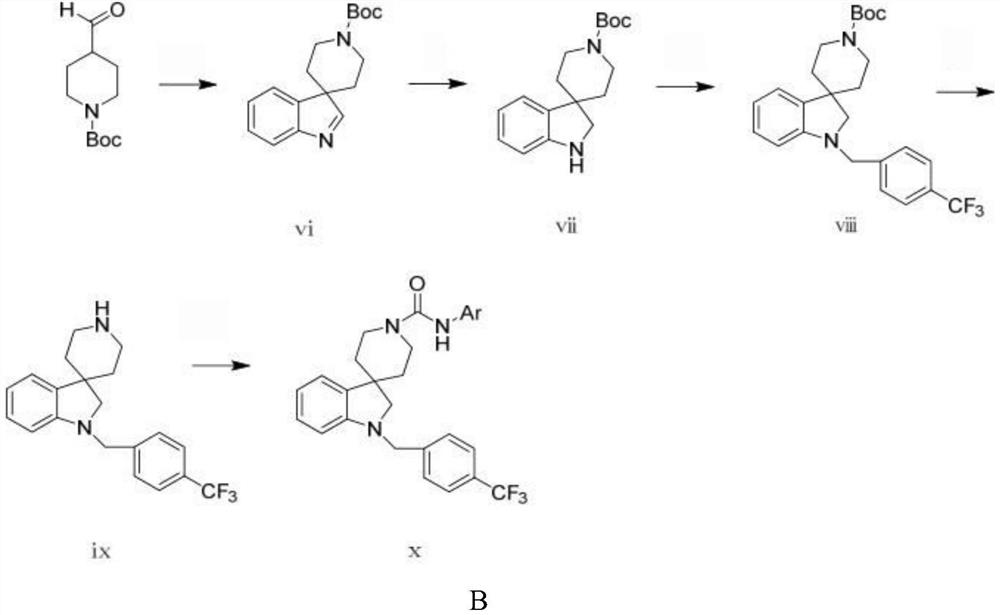
Image
Examples
Embodiment 1
[0050] Example 1: N-(2,4-dimethylphenyl)-1-propionylspirocycle [indoline-3,4'-piperidine]-1'-carboxamide represented by formula (1) preparation
[0051]
[0052] The preparation method includes the following steps:
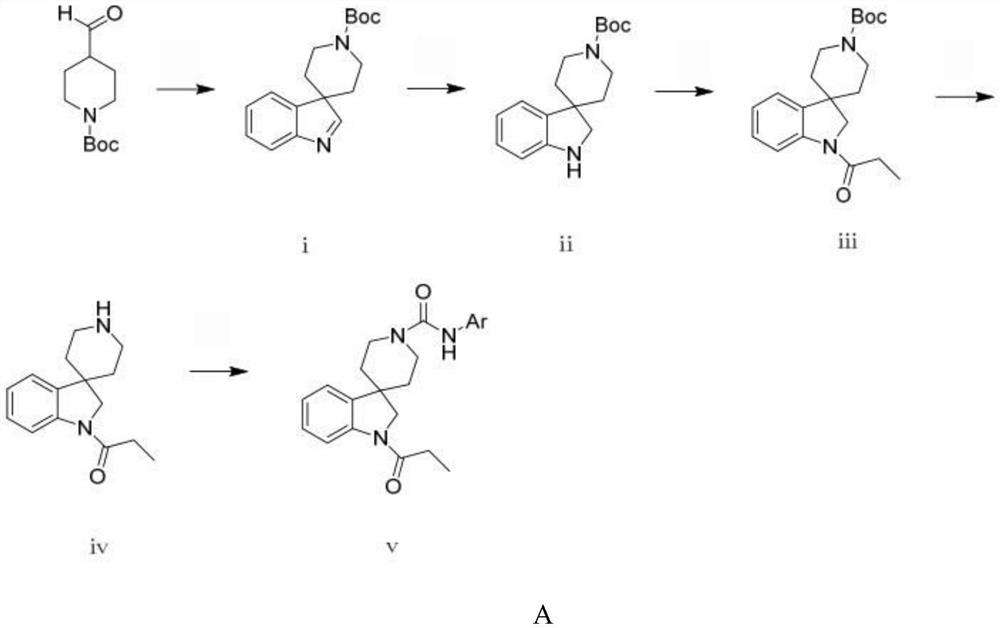
[0053] (a) Preparation of spiro[indole-3,4'-piperidine]-1'-carboxylate tert-butyl ester
[0054] Dissolve 10 g (0.0469 mol) of 1-Boc-4-piperidinal in 468 mL of dichloromethane to prepare a 0.1 mol / L solution, add 4.615 mL (0.0469 mol) of phenylhydrazine under ice bath, and stir for 10 min, and slowly add trifluorocarbon 6.964 mL (0.0938 mol) of acetic acid was reacted at room temperature overnight, 100 mL of saturated aqueous sodium bicarbonate solution was added to quench the reaction, extracted with dichloromethane 3 times, each using 100 mL, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain spiro[indole] -3,4'-Piperidine]-1'-carboxylic acid tert-butyl ester is a brown oily liquid.
[0055] (b) Preparation of spiro[indo...
Embodiment 2
[0064] Example 2: N-(2,5-dimethylphenyl)-1-propionylspirocycle [indoline-3,4'-piperidine]-1'-carboxamide represented by formula (2) preparation
[0065]
[0066] Substitute 2,4-dimethylaniline in step (g) of Example 1 with 137 μL (1.101 mmol) of 2,5-dimethyl-aniline, and refer to the preparation method in Example 1 for other steps to obtain compound ( 2) to obtain a white solid, yield: 63%. The experimental data is as follows:
[0067] C 24 H 29 N 3 O 2 , White solid (47.6% yield), mp=182.5-184.5℃; 1 H NMR (300MHz, DMSO-d 6 )δppm 8.11(d,J=8.0Hz,1H,NH),8.07(s,1H,Ar-H),7.31-7.14(m,2H,Ar-H),7.04(q,J=7.3Hz,3H , Ar-H), 6.87(d, J=7.5Hz, 1H, Ar-H), 4.12(d, J=13.8Hz, 2H, Piperidine), 4.07(s, 2H, pyrrolidine), 3.00(t, J =12.4Hz, 2H, Piperidine), 2.64-2.52(m, 2H, CH 2 ),2.25(s,3H,Ar-CH 3 ),2.15(s,3H,Ar-CH 3 ),1.87-1.71(m,2H,Piperidine),1.63(d,J=12.8Hz,2H,Piperidine),1.09(t,J=7.2Hz,3H,CH 3 ).
Embodiment 3
[0068] Example 3: Preparation of N-(2,5-dichlorophenyl)-1-propionylspiro[indoline-3,4'-piperidine]-1'-carboxamide represented by formula (3)
[0069]
[0070] Substitute 2,4-dimethylaniline in step (g) of Example 1 with 83 μL (1.167 mmol) of 2,5-dichloro-aniline, and refer to the preparation method in Example 1 for other steps to obtain compound ( 3) to obtain a white solid, yield: 61%. The experimental data is as follows:
[0071] C 22 H 23 Cl 2 N 3 O 2 , White solid (47.6% yield), mp=174.5-176.5℃; 1 H NMR (300MHz, DMSO-d 6 )δppm 8.39(s,1H,NH),8.11(d,J=7.9Hz,1H,Ar-H),7.68(t,J=2.8Hz,1H,Ar-H),7.50(d,J=8.6 Hz,1H,Ar-H),7.22-7.19(m,J=15.3,13.1,8.3Hz,3H,Ar-H),7.02(t,J=7.4Hz,1H,Ar-H),4.14(d ,2H,Piperidine),4.07(s,2H,pyrrolidine),3.06(t,J=12.5Hz,2H,Piperidine),2.64-2.51(m,2H,CH 2 ),1.90-1.73(m,2H,Piperidine),1.65(d,J=12.9Hz,2H,Piperidine),1.08(t,J=7.2Hz,3H,CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com