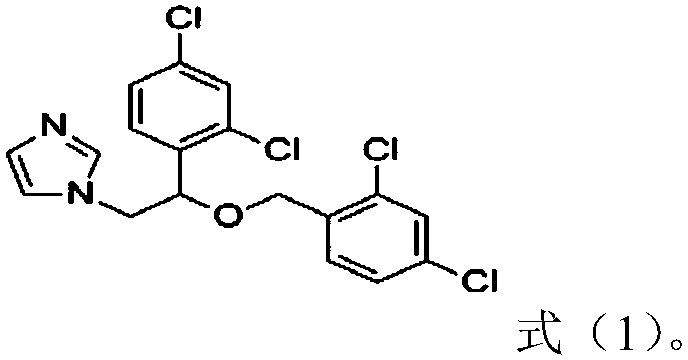
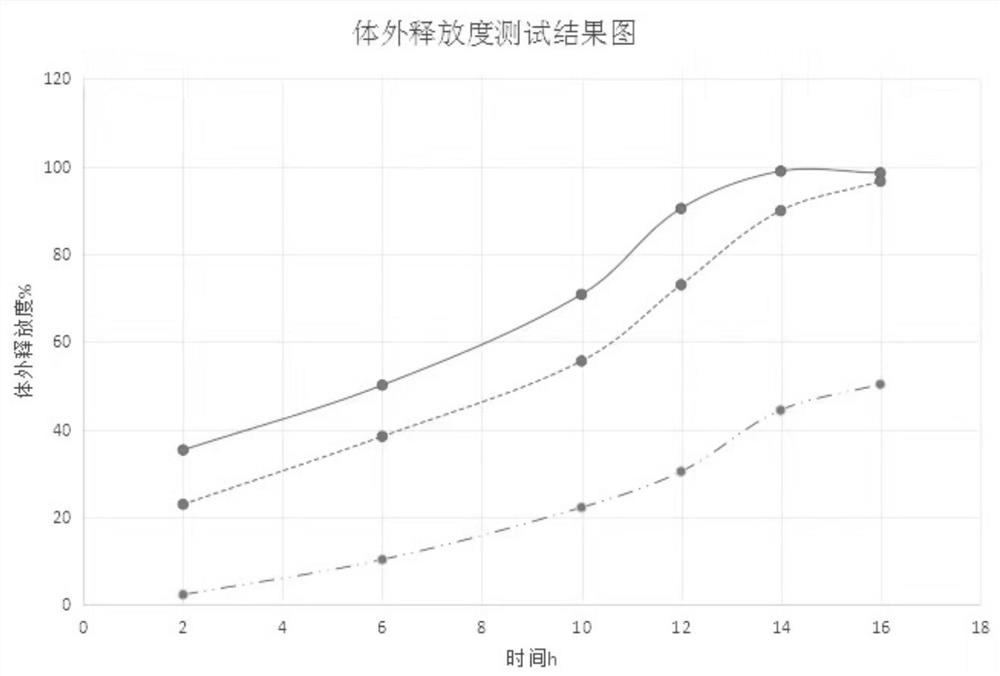
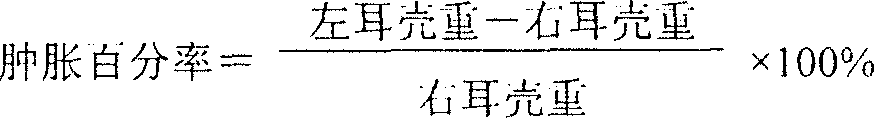Patents
Literature
44 results about "Micronazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nanometer miconazole nitrate emulsion medicine and its prepn process
InactiveCN1931164AImprove solubilityImprove bioavailabilityOrganic active ingredientsAntimycoticsPolymer chemistryOil phase
The nanometer miconazole nitrate emulsion medicine consists of surfactant, oil, miconazole nitrate and distilled water. Its preparation process includes the following steps: weighing surfactant with or without co-surfactant; calculating HLB value and selecting oil phase for reaching emulsifying HLB value near that of the surfactant phase; changing the ratio between the surfactant phase and the oil phase regularly; adding dimethyl sulphoxide with dissolved miconazole nitrate into surfactant through stirring at 20-25 deg.c; adding distilled water slowly to form clear and flowing O / W type nanometer emulsion liquid. The nanometer miconazole nitrate emulsion has high skin permeability, no contamination to clothing, high dissolubility of miconazole nitrate, raised bioavailability of miconazole nitrate, delayed metabolism time and wide medicine market foreground.
Owner:NORTHWEST A & F UNIV
Microbial limit detection method of miconazole nitrate suppository
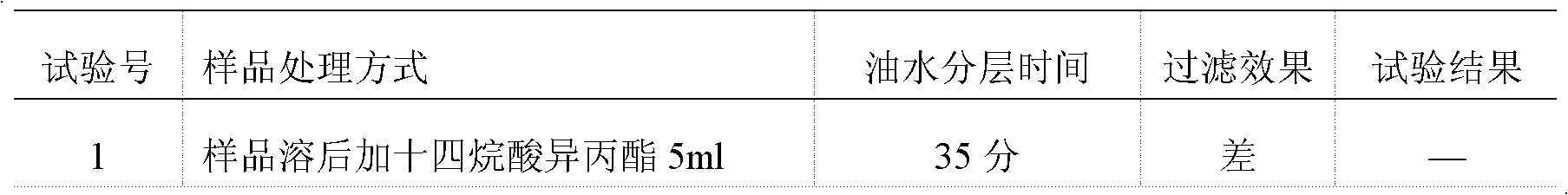
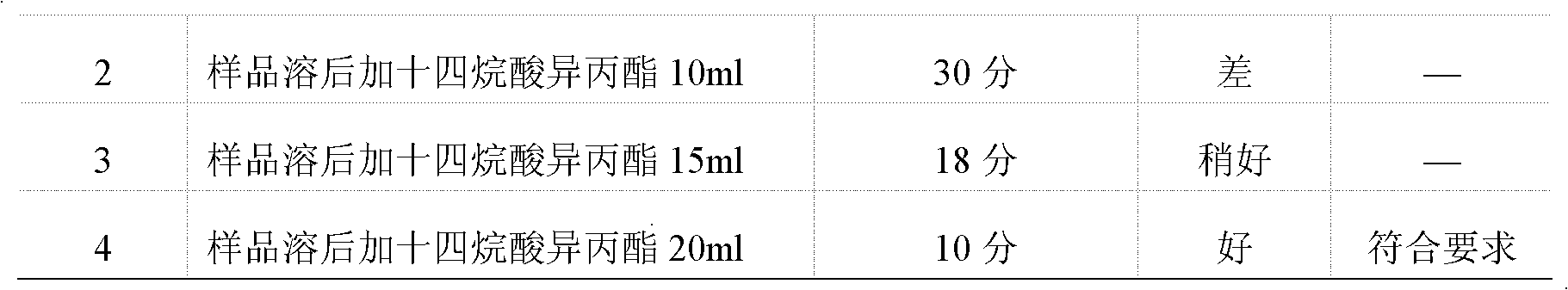
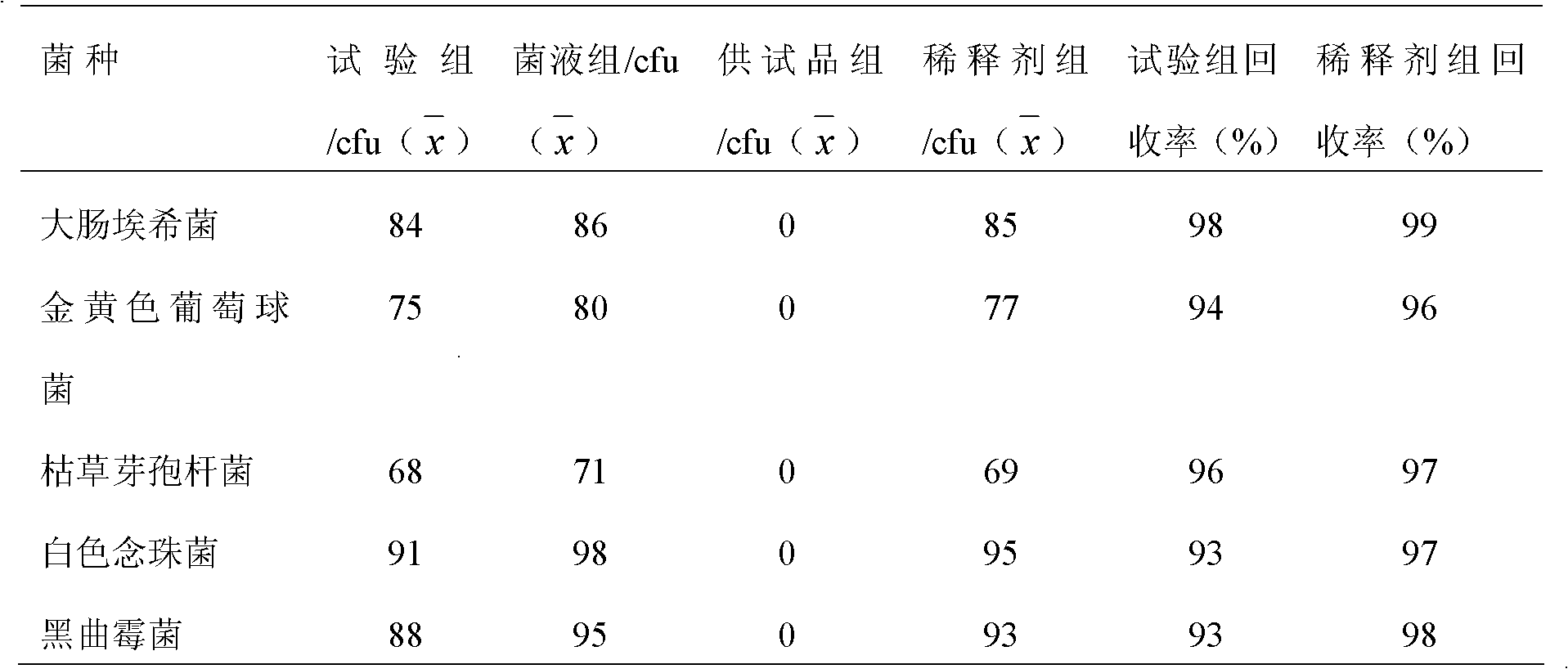
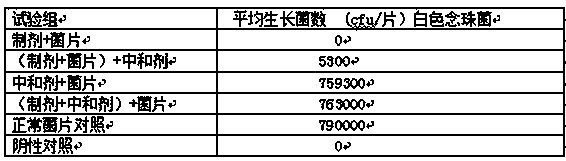
InactiveCN102586393AGood antibacterial effectPromote stratificationMicrobiological testing/measurementMicroorganism based processesFiltrationStaphyloccocus aureus
The invention discloses a microbial limit detection method of miconazole nitrate suppository. The method comprises dissolving sample with sodium chloride-peptone buffer solution (pH 7.0) containing 10 vol% polysorbate 80 (Tween 80), adding 20ml of isopropyl myristate, and washing with aqueous solution containing 0.1 vol% polysorbate 80 and 0.1 wt% peptone. Membrane filtration method is adopted for bacteria, mildew, yeast, Staphyloccocus aureus and Monilia albican, with recovery rate all above 70%. Culture medium dilution method is adopted to detect Pseudomonas Aeruginosa. The addition of isopropyl myristate can accelerate fat / water layer separation, shorten experiment time, stabilize flow rate, reduce test cost, and achieve strong operability.
Owner:SHAANXI INST FOR FOOD & DRUG CONTROL
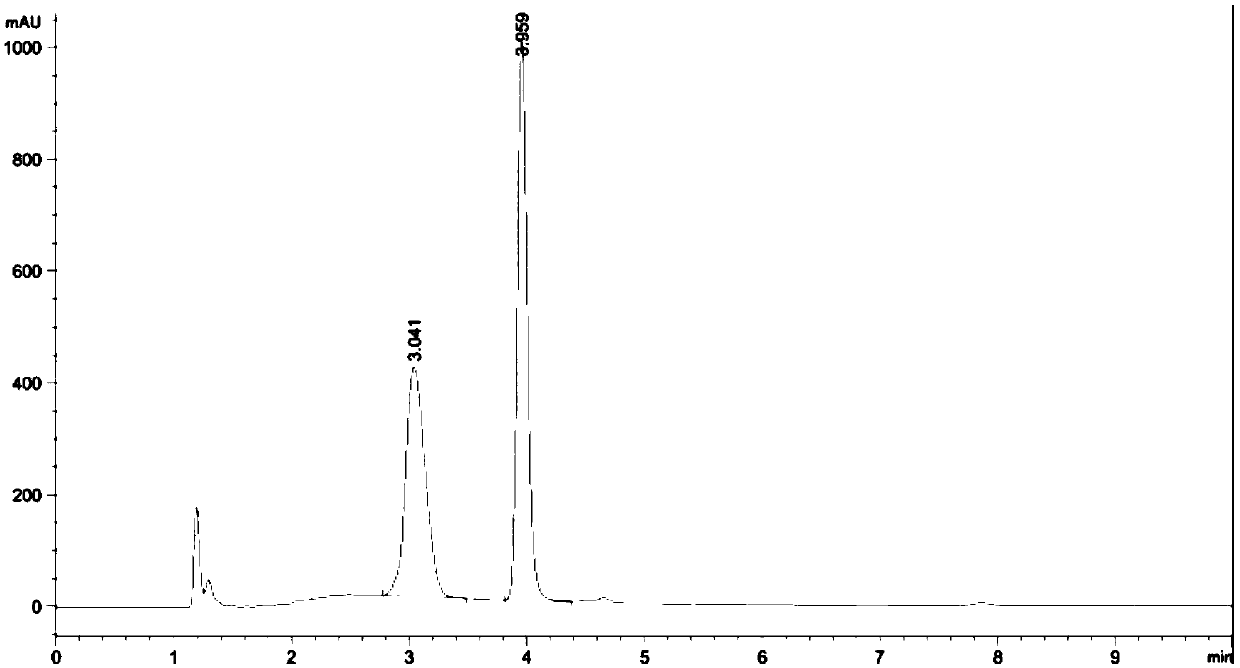
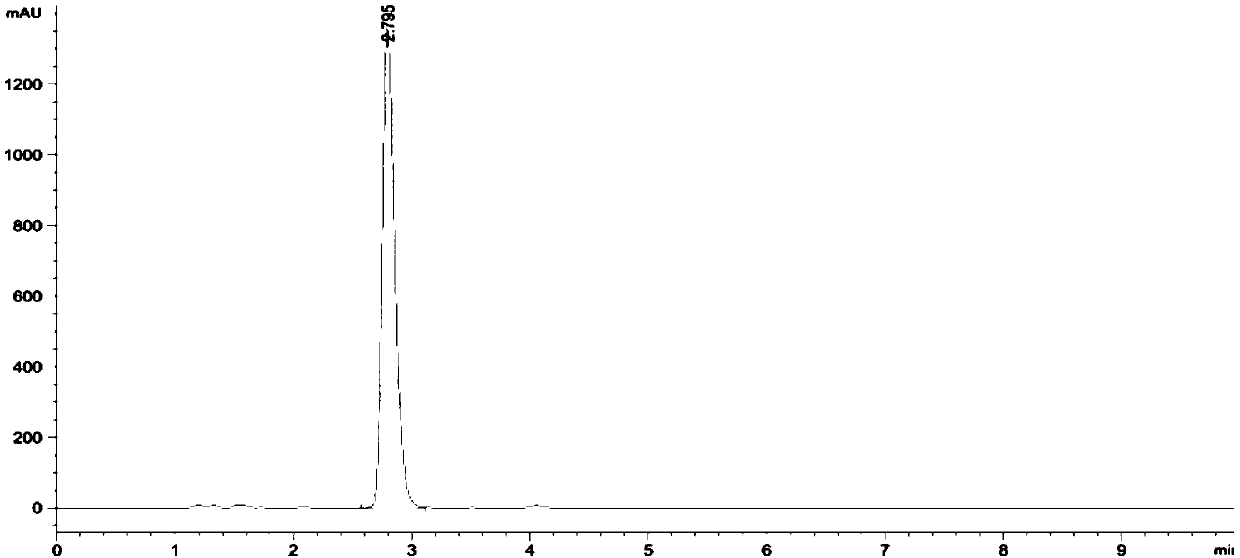
Method for determining contents of miconazole nitrate and triamcinolone acetonide in Qumicin cream
InactiveCN1614410AImprove quality controllabilityEasy to separateComponent separationPreparing sample for investigationMicronazoleNitrate
A method for determining content of triamcinolone acetonide acetate and micronazole nitrate in qumixing emulsifiable paste utilizes high efficiency liquid plase external standard method to determine simultaneously out content of triamcinolone acetonide acetate and miconazole nitrate in qumixing emulsifiable paste.
Owner:GUANGZHOU BAIYUSN HUTCHISON WHAMPOA CHINESE MEDICINE
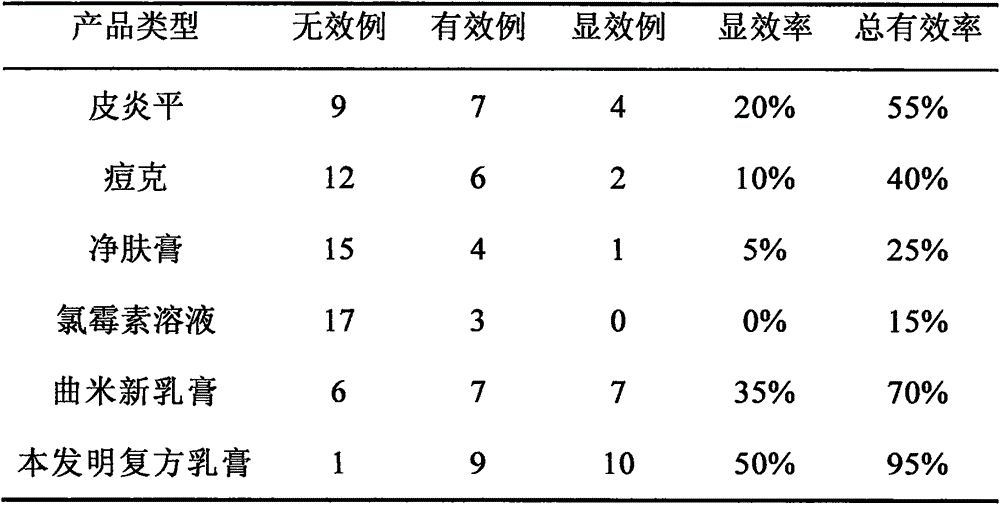
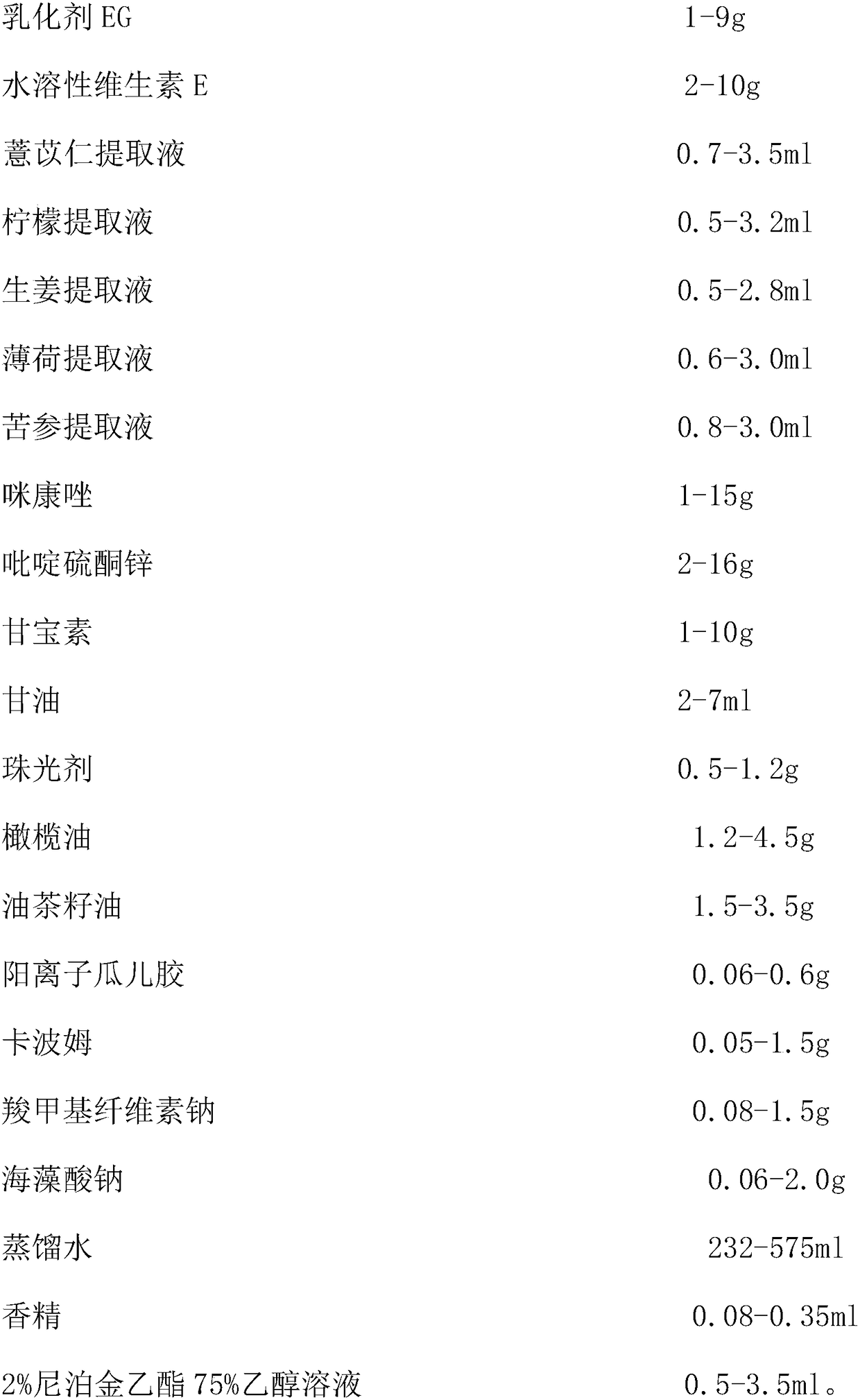
Antifungal medicine composition and its preparing method and use
ActiveCN1985883AEliminate side effectsImprove the problem of unstable curative effectAntimycoticsHydroxy compound active ingredientsAntifungalWestern medicine
The antifungal medicine composition is medicine prepared with the materials including snake oil 50-100 weight portions, Miconazole nitrate 25-50 weight portions, flavescent sophora root 20-50 weight portions, pearl powder 1-10 weight portions, menthol 1-5 weight portions, and borneol 1-5 weight portions. The antifungal medicine composition as one compound Chinese and Western medicine preparation contains effective antifungal components, and components for clearing away heat and toxic material, eliminating swelling, relieving pain, astringing and promoting granulation. It is used in treating tinea corporis, tinea cruris, tinea manuum, tinea pedis, etc caused by mycotic infection, and has stable treating effect and little side effect.
Owner:JIANGSU KANION PHARMA CO LTD
Lotion for preventing and killing fungi and bacteria on dog/ cat body surface and preparation method thereof
InactiveCN102526562ALow toxicityGood antibacterial effectOrganic active ingredientsAntisepticsMedicinal herbsChlorhexidine Acetate
The invention relates to lotion combining Chinese and Western medicines and used for killing, preventing and controlling fungal and bacterial diseases on dog / cat body surfaces. The lotion consists of bactericidal and bacteriostatic Chinese medicinal herbs such as pseudolarix, smilax glabra, common cnidium fruit, sophora flavescens, cortex dictamni, phellodendron, Szechuan pepper and dried alum, broad-spectrum efficient miconazole fungicide and efficient chlorhexidine acetate bactericide according to a scientific formula. By combining the Chinese and Western medicines, the bactericidal and bacteriostatic effect is enhanced, the antibacterial spectrum is enlarged, the medicament tolerance produced by long-term use is avoided, and the toxicity of blindly used chemicals is reduced. The lotion for pets has wide spectrum for killing fungi and bacteria, high safety and low toxic or side effects, and can be used for a long term. The formulation can be prepared into spray, lotion, liniment and externally applied ointment by adopting pharmaceutically medicament preparation technologies.
Owner:丁国成
Detection method of compound preparation containing compound Zingiber corallinum Hance solution and urea miconazole ointment
ActiveCN102788863AImprove quality testing standardsKeep healthyComponent separationPreparing sample for investigationMicronazoleClinical efficacy
The invention discloses a detection method of a compound preparation containing a compound Zingiber corallinum Hance solution and a urea miconazole ointment. The detection method comprises items of characters, inspection, identification and content determination. The identification comprises a qualitative identification, a thin-layer chromatography identification with a contrast of Zingiber corallinum Hance reference medicine, an efficient liquid chromatography identification with contrasts of a salicylic acid reference substance, a Zingiber corallinum Hance reference substance and a miconazole nitrate reference substance, and a gas chromatography identification with a contrast of a borneol reference substance. The content determination is for determining content of Zingiber corallinum Hance, salicylic acid, borneol, urea and miconazole nitrate in the preparation. The invention is scientific and reasonable, has high accuracy and strong feasibility, improves stability of the compound preparation containing compound Zingiber corallinum Hance solution and urea miconazole ointment, and effectively controls quality of the compound preparation containing compound Zingiber corallinum Hance solution and urea miconazole ointment, thus ensuring clinical efficacy of the compound preparation.
Owner:GUIZHOU JINQIAO PHARMA
Method for producing ergothioneine
InactiveUS20170211107A1Raise the level of fermentationImprove production yieldFungiCulture processBetaineArginine
The present disclosure relates to an improved method for producing ergothioneine, comprising the steps of: (a) inoculating Pleurotus ostreatus strain CGMCC No. 6232 into a seed medium, and culturing it to prepare a seed liquor, wherein the seed medium uses soybean cake powder as nitrogen source; and (b) inoculating the seed liquor into a fermentation basal medium, and then culturing it to obtain a fermentation broth of Pleurotus ostreatus mycelia. Further, any one or more members selected from NH4Cl, NH4NO3, NaCl, polyethylene glycol, folic acid, vitamin B1 (VB1), indolebutyric acid, citric acid, pyruvic acid, arginine, lysine, leucine, aspartic acid, glutamic acid, betaine, histidine, cysteine, methionine, tween, span, chitosan, Fluconazole, Miconazole, Ketoconazole, ethylenediaminetetraacetic acid (EDTA), isopropyl alcohol and dimethyl sulfoxide are added into the fermentation basal medium.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI +1
Acinetobacter zjph1806 and its application in the preparation of miconazole chiral intermediates
The invention discloses an Acinetobacter ZJPH1806 and its application in the preparation of a chiral intermediate of miconazole. (R)-2-chloro-1 is prepared by asymmetric reduction of 2,2',4'-trichloroacetophenone ‑(2,4‑chlorophenyl)ethanol, the product obtained is of high optical purity. In the pH 7.6 phosphate buffer system, with glycerol as the auxiliary substrate, 2g / L substrate was added to react for 26h, the yield of R-type product was 83.2%, and the e.e. value was >99.9%.
Owner:ZHEJIANG UNIV OF TECH
Acinetobacter sp. ZJPH1806 and application thereof to preparation of miconazole chiral intermediate
ActiveCN110016444AHigh optical purityBacteriaMicroorganism based processesGlycerolMedicinal chemistry
The invention discloses acinetobacter sp. ZJPH1806 and an application thereof to preparation of a miconazole chiral intermediate. 2,2',4'-trichloroacetophenone is subjected to asymmetric reduction toprepare (R)-2-chlorine-1-(2,4-chlorophenyl) ethanol, and obtained products are high in optical purity. In a phosphate buffer liquid system of which the pH is 7.6, glycerine is used as an auxiliary substrate, 2g / L of a substrate is added, a reaction is performed for 26h, the yield of R-type products is 83.2%, and e.e. value is greater than 99.9%.
Owner:ZHEJIANG UNIV OF TECH
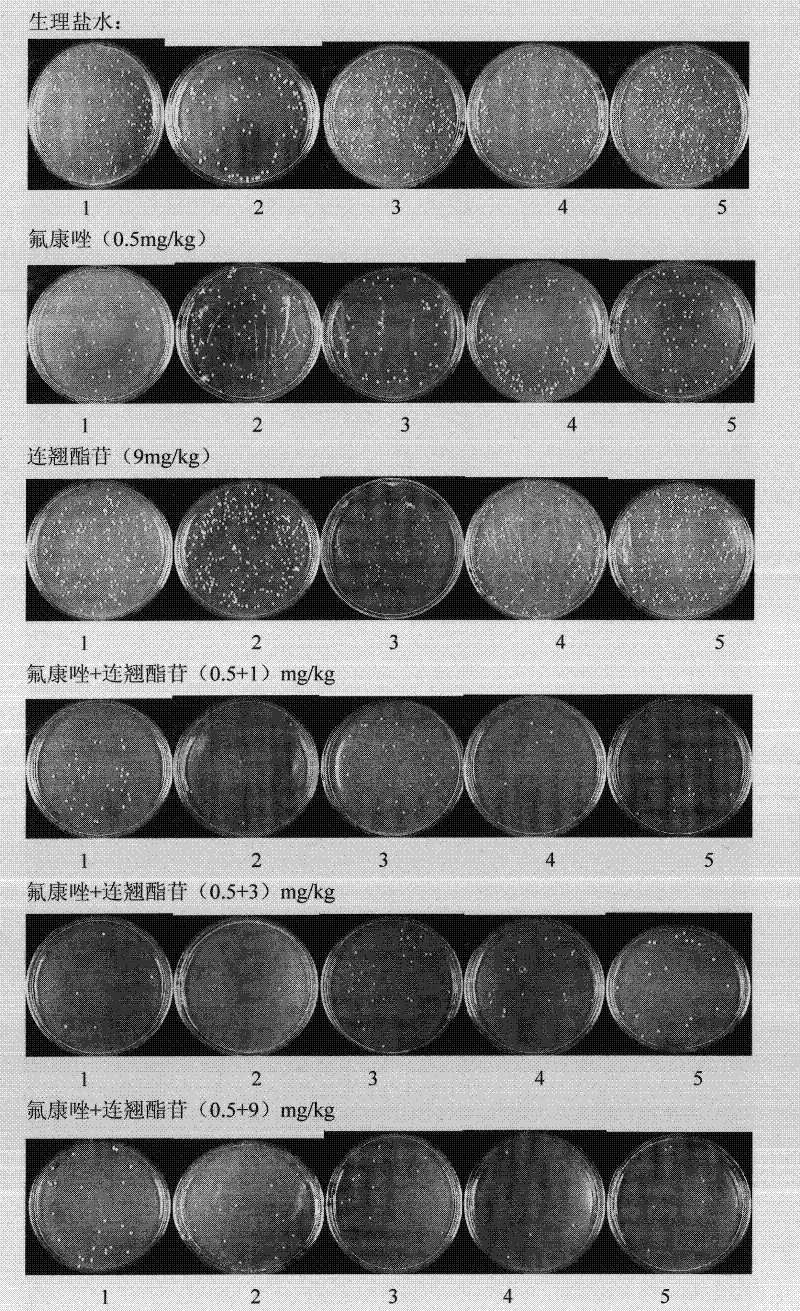
Use of forsythiaside as synergist of antifungal agents
InactiveCN101816666AReduce doseEnhanced inhibitory effectOrganic active ingredientsAntimycoticsSide effectFluconazole
The invention relates to the technical field of medicines and discloses a new use of forsythiaside as a synergist of antifungal agents. In vivo and in vitro experiments show that the forsythiaside can be combined with the antifungal agents, such as fluconazole, itraconazole, miconazole, ketoconazole and the like, thereby improving the treatment effect of superficial and deep fungal infection with different degrees, leading the antifungal agents to recover the role to fungi with drug resistance and further using the forsythiaside as the synergist of the antifungal agents. The invention opens up the new use of the forsythiaside, and the use of the forsythiaside as the synergist of the antifungal agents can not only improve the antifungal role of drugs, but also lead the antifungal agents to recover the role to the fungi with drug resistance under the situations that the clinical drug resistance of the fungi is increasingly common and the drug resistance degree is increasingly serious, and further reduce the dosage of the antifungal agents, thereby saving medical expenses for patients and reducing toxicity and side effects of the drugs.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Production technology of miconazole nitrate vaginal tablets
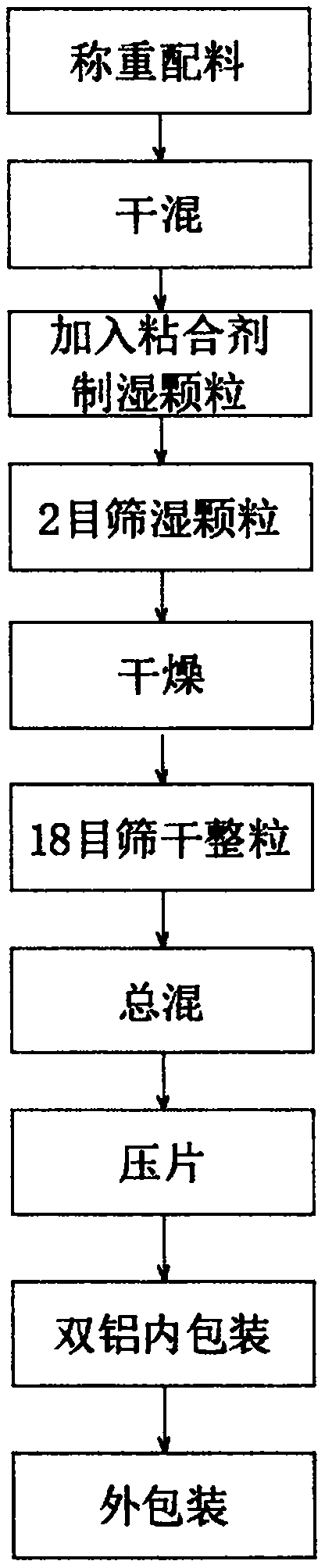
InactiveCN110237042AIncrease profitImprove production efficiencyOrganic active ingredientsAntimycoticsPharmacyDry mixing
The invention provides a production technology of miconazole nitrate vaginal tablets, and relates to the technical field of a medicine making technology. The production technology of miconazole nitrate vaginal tablets comprises the following steps of performing weighing and blending, performing dry mixing to obtain wet granules, performing drying, performing granule trimming, performing total mixing, performing tabletting, performing packaging, and performing warehousing: performing packaging, performing total inspection, and performing warehousing. The miconazole nitrate vaginal tablets are produced through an assembly line of whole automated equipment, so that the making efficiency of the whole products can be improved, the yield of unit time can be increased, enterprise profits are increased, quality control is performed for products produced through multiple working procedures, the quality of products produced through a single working procedure can be guaranteed, control on the quality of finished products is realized, the content of defectives in the pharmacy process is reduced, and the whole rate of finished products in the whole pharmacy technological process is increased.
Owner:JINAN LIMIN PHARMA
Western medicine ointment for treating tinea of feet and hands
InactiveCN106581636AGood antibacterial effectAntipruriticAntimycoticsHydroxy compound active ingredientsGlycerolAugmented Ointment
The invention discloses a western medicine ointment for treating tinea of feet and hands. The western medicine ointment is prepared from the following raw materials in parts by weight: 2-5 parts of miconazole nitrate cream, 2-5 parts of mometasone furoate gel, 5-10 parts of glycerol, 5-8 parts of ocean fish oligopeptides, 0.2-0.5 parts of vitamin E, 2-5 parts of Loratadine, 2-5 parts of Amprenavir, 0.01-0.05 parts of an anti-inflammation preservative, 1-3 parts of radix glycyrrhizae, 0.1-0.5 parts of flavones, 0.5-2 parts of snake oil, 0.01-0.05 parts of chondroitin sulfate, 1-2 parts of a terbinafine hydrochloride solution, and 10-20 parts of honey. The western medicine ointment achieves a synergistic effect by compounding the raw materials, has good effects of inhibiting bacteria, relieving itching and killing the bacteria, and can effectively treat the tinea of feet and hands; the western medicine ointment has the advantages of difficult relapse, low drug dependence, short treatment course and radical treatment; and the western medicine ointment also has the curative effects of activating blood to remove stasis, diminishing inflammation to relieve pain, and preventing infection.
Owner:ZHENGZHOU ZHENGXIAN PHARMA CO LTD
Method for detecting 15 forbidden azoles in children cosmetics
PendingCN114594183AMeet the banned ingredient monitoring requirementsMulti-sensitivityComponent separationMicronazoleLiquid chromatography mass spectroscopy
The invention relates to a method for detecting 15 forbidden azoles in children cosmetics. According to the method, 15 azole substances such as econazole, clotrimazole, ketoconazole, fluconazole, miconazole, neoconazole, benznidazole, metronidazole, metronidazole, ornidazole, tinidazole, isometronidazole, lornidazole, hydroxy metronidazole and bifonazole in the children cosmetics can be efficiently and rapidly detected by adopting a liquid chromatography-mass spectrometry method. The detection method fills the blank of children cosmetic detection standards, is high in pertinence, high in sensitivity and multiple in detection types, and protects children cosmetics.
Owner:GUANGDONG TESTING INST OF PROD QUALITY SUPERVISION
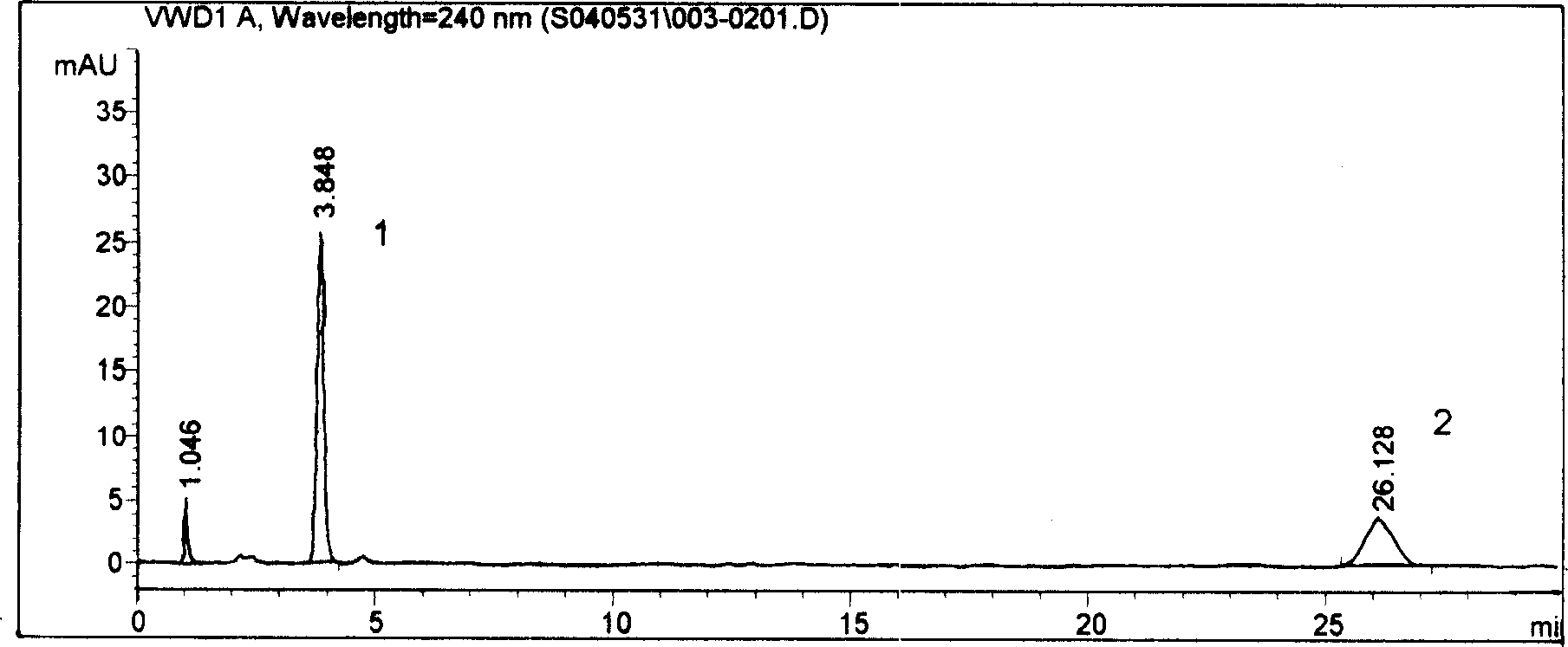
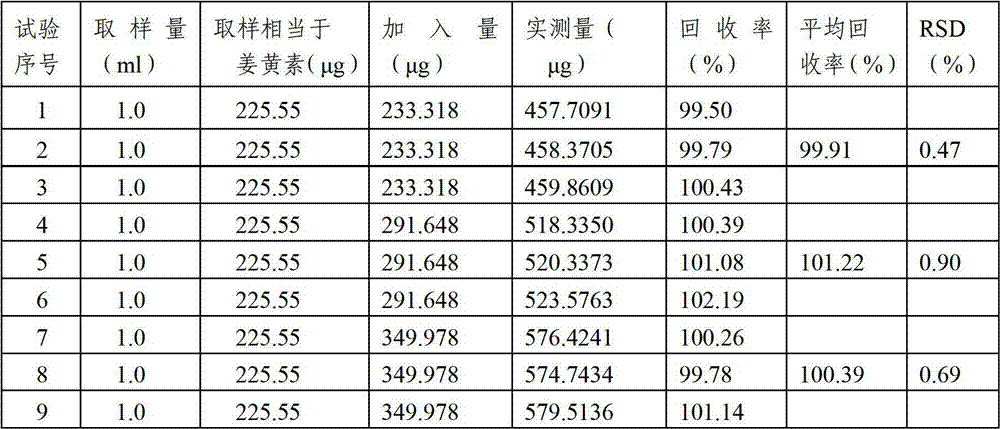
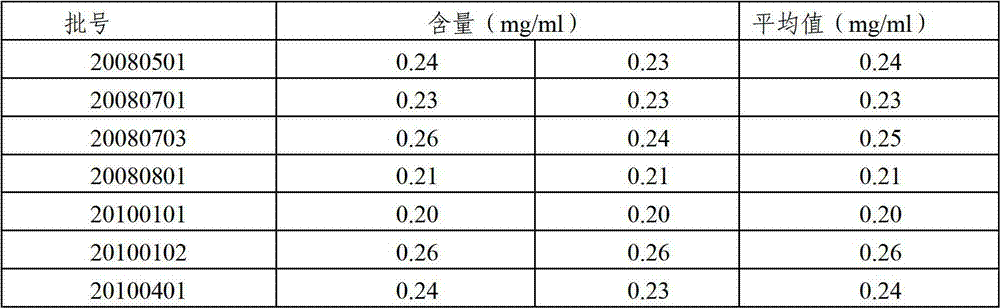
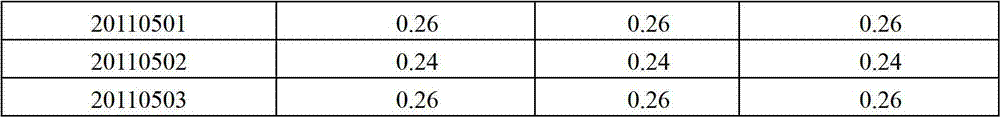
Content detecting method of miconazole nitrate
The invention relates to a content detecting method of miconazole nitrate. A to-be-detected sample is diluted into a test solution with the content of the miconazole nitrate being 0.02 mg / mL-0.2 mg / mLthrough a solvent; 10 [mu]L-20 [mu]L of the test solution is taken to be injected into a high performance liquid chromatograph to be detected, and the content of the miconazole nitrate in the to-be-detected sample is obtained; and the chromatographic conditions during high performance liquid chromatographic detection are that a chromatographic column takes octadecylsilane chemically bonded silicaas a filler, a flowing phase is prepared from acetonitrile, a phosphate solution and triethylamine according to the volume ratio of (35 to 50):(50 to 65):1, the pH value of the flowing phase is 3+ / -0.1, the concentration of the phosphate solution is 0.02 mol / L-0.08 mol / L, the flowing speed is 0.5 mL / min-1.5 mL / min, the column temperature is 25 DEG C-35 DEG C, the detection wavelength is 210 nm-240 nm, and the solvent and the flowing phase are the same in composition. The condition response value of the method is high, peak appearing of the miconazole nitrate is quick, the content of the miconazole nitrate can be measured quickly, the working efficiency of detection is improved, and the quality of a product is controlled advantageously.
Owner:FOSHAN NANHAI EASTERN ALONG PHARMA CO LTD
Drug for treating dermatoses and preparation method and application thereof
InactiveCN106074572ASmall particle sizeLow viscosityOrganic active ingredientsAntimycoticsNeomycin SulfateRemove blood
The invention discloses a drug for treating dermatoses. The drug is prepared from, by weight, 55-120 parts of chloramphenicol, 120-200 parts of propylene glycol, 50-90 parts of water for injection, 0.5-1.5 parts of chlorpheniramine maleate, 200-280 parts of white vaseline, 160-220 parts of glycerol, 1.5-3 parts of miconazole nitrate, 0.2-0.5 part of triamcinolone acetonide acetate, 220-300 parts of natural fatty alcohol and 0.5-0.9 part of neomycin sulfate. The drug has the effects of clearing away heat and toxic materials, promoting blood circulation to remove blood stasis, purging fire and cooling blood, relieving swelling and pain and resisting bacteria and viruses, is used for treating the dermatoses such as acnes, pachulosis, sunburn, dermatophytosis and mosquito bites and has the advantages that the treatment effect is good and is quickly achieved, the cure rate is high, cost is low, no irritation is generated to skin, and scars are not prone to be left.
Owner:梁东荣
Method for producing ergothioneine by using soybean cake powder as nitrogen source in a seed medium
The present disclosure relates to an improved method for producing ergothioneine, comprising the steps of: (a) inoculating Pleurotus ostreatus strain CGMCC No.6232 into a seed medium, and culturing it to prepare a seed liquor, wherein the seed medium uses soybean cake powder as nitrogen source; and (b) inoculating the seed liquor into a fermentation basal medium, and then culturing it to obtain a fermentation broth of Pleurotus ostreatus mycelia. Further, any one or more members selected from NH4Cl, NH4NO3, NaCl, polyethylene glycol, folic acid, vitamin B1 (VB1), indolebutyric acid, citric acid, pyruvic acid, arginine, lysine, leucine, aspartic acid, glutamic acid, betaine, histidine, cysteine, methionine, tween, span, chitosan, Fluconazole, Miconazole, Ketoconazole, ethylenediaminetetraacetic acid (EDTA), isopropyl alcohol and dimethyl sulfoxide are added into the fermentation basal medium.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI +1
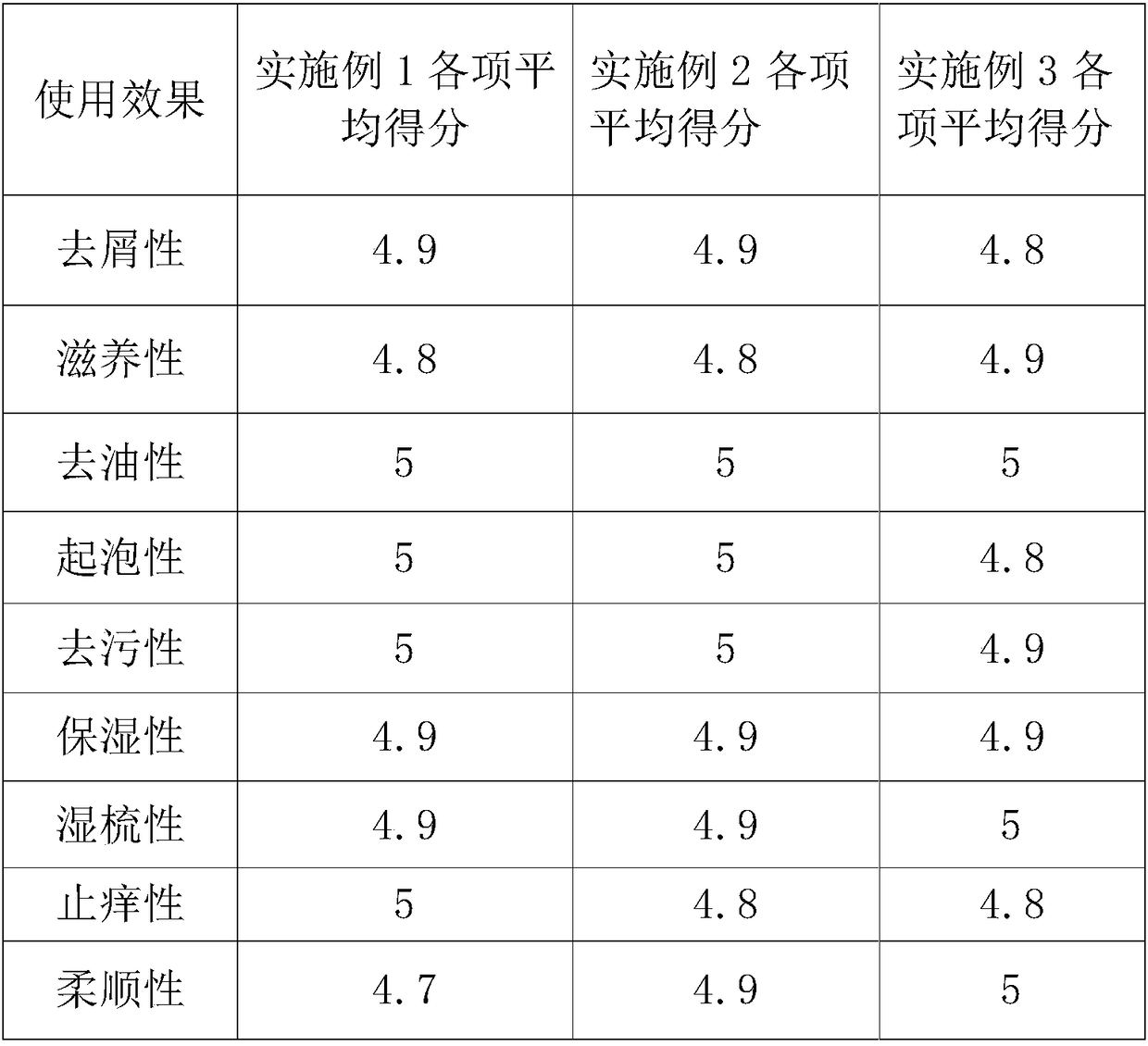
Oil-removing, anti-dandruff, anti-itch and moisturizing shampoo and preparation method thereof
ActiveCN105853300BHas anti-inflammatory and analgesic effectsAnti agingCosmetic preparationsAntimycoticsHair rootsGlycerol
The invention discloses traditional Chinese medicine dandruff removing, degreasing, itching relieving and moistening cream shampoo and a preparation method thereof. The cream shampoo is prepared from sodium laureth sulfate, sodium N-lauroylglutamate, MES, GOC, EG, water-soluble vitamin E, an amino acid surfactant, a coix seed extracting solution, a lemon juice extracting solution, a fresh ginger extracting solution, a herba menthae extracting solution, a radix sophorae flavescentis extracting solution, a miconazole aqueous solution, zinc pyrithione, climbazol, glycerol, a pearling agent, ethylparaben, olive oil, camellia oleosa seed oil, cationic guar gum, Carbomer gel, sodium carboxymethylcellulose, sodium alginate gel, distilled water and essence. The cream shampoo is mild in nature and high in safety and can remove grease of hair, effectively relieve itching, inhibit generation of dandruff, supplement nutrient to the hair, nourish and moisten the hair, improve blood circulation of the scalp, promote metabolism, strengthen the hair roots, prevent epilation, promote growth of new hair and enable the hair to be soft, smooth, beautiful and bright.
Owner:汕头市深特宝洁实业有限公司
Treatments for free-living amoebic infections
ActiveUS10806741B2Effective treatmentReduce adverse side effectsPharmaceutical delivery mechanismPhosphorous compound active ingredientsAmoebic encephalitisSappinia
Methods of treating infections caused by free-living amoeba are disclosed. The methods generally include systemic administration of an effective amount of miltefosine, such as an oral or intravenous formulation, and optionally local administration of an effective amount of miltefosine, such as a topical formulation of miltefosine. The methods may further include administration of one or more secondary agents. Exemplary secondary agents include steroids, polyhexamethylene biguanide (PHMB), chlorhexidine, propamidine isethionate, dibromopropamidine isethionate, neomycin, paromomycin, polymyxin B, clotrimazole, ketoconazole, miconazole, and itraconazole. The methods may be used to treat patients with infections caused by a free-living amoeba such as Naegleria fowleri, Balamuthia mandrillaris, Sappinia diploidea, and Acanthamoeba species. Exemplary infections include Acanthamoeba keratitis, granulomatous amoebic encephalitis, cutaneous acanthamoebiasis, primary amoebic meningoencephalitis, Sappinia amoebic encephalitis, or a disseminated disease associated with a free-living amoeba.
Owner:PROFOUNDA
Children eczema ointment and preparation method thereof
InactiveCN111840280AInhibit synthesisSafe and non-irritating to useHydrocarbon active ingredientsAntimycoticsMicronazoleCell membrane
The invention discloses a children eczema ointment and a preparation method thereof, and belongs to the field of eczema medicines. According to the children eczema ointment provided by the invention,miconazole nitrate can inhibit synthesis of fungal cell membranes and kill fungi, all the medicinal components cooperate with one another to jointly achieve the effects of relieving itching, diminishing inflammation, inhibiting bacteria and killing bacteria, and the ointment particularly has an obvious effect on fungal infection and eczema infection, is safe and non-irritant to use, and is suitable for children to take externally.
Owner:徐大伟
Use of forsythiaside as synergist of antifungal agents
InactiveCN101816666BImprove antifungal effectSmall toxicityOrganic active ingredientsAntimycoticsSide effectItraconazole
The invention relates to the technical field of medicines and discloses a new use of forsythiaside as a synergist of antifungal agents. In vivo and in vitro experiments show that the forsythiaside can be combined with the antifungal agents, such as fluconazole, itraconazole, miconazole, ketoconazole and the like, thereby improving the treatment effect of superficial and deep fungal infection withdifferent degrees, leading the antifungal agents to recover the role to fungi with drug resistance and further using the forsythiaside as the synergist of the antifungal agents. The invention opens up the new use of the forsythiaside, and the use of the forsythiaside as the synergist of the antifungal agents can not only improve the antifungal role of drugs, but also lead the antifungal agents torecover the role to the fungi with drug resistance under the situations that the clinical drug resistance of the fungi is increasingly common and the drug resistance degree is increasingly serious, and further reduce the dosage of the antifungal agents, thereby saving medical expenses for patients and reducing toxicity and side effects of the drugs.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Application of miconazole to preparation of tumor resistant medicines
InactiveCN110917192AAntitumor effect is obviousStable in natureOrganic active ingredientsAntineoplastic agentsPancreas CancersMicronazole
The invention belongs to the field of pharmaceuticals, and discloses an application of miconazole to preparation of tumor resistant medicines. Tumors include pancreatic cancer, liver cancer, lung cancer and stomach cancer. The miconazole is an artificially-synthesized 1-phenylethyl imidazole derivative, and is a broad-spectrum fungus resistant medicine. The miconazole is used as a new tumor resistant medicine or an auxiliary component thereof for development, and is obvious in tumor resistant effects. Therefore, the miconazole is used for resisting tumors, and has great application prospects.
Owner:EAST CHINA NORMAL UNIV +1
HPLC detection method of related substances in Qumixin cream
The invention belongs to the field of chemical detection, and in particular relates to a high performance liquid chromatography (HPLC) method for detecting triamcinolone acetonide acetate and miconazole nitrate related substances in Qumixin cream. The method comprises the following steps: (1) testing the chromatographic condition and system suitability; (2) preparing a solution; (3) determining, and the like. The HPLC method for detecting the triamcinolone acetonide acetate and the miconazole nitrate related substances in the Qumixin cream is simple to operate, better in degree of separation,many in detected related substances and high in detection efficiency, and has good specificity, precision and accuracy; compared with the prior art, the method can detect more impurities, and can moreeffectively control the related substances in the Qumixin cream.
Owner:GUANGDONG SHUNFENG PHARMA
Oral sustained-release gel for treating candidiasis and preparation method of oral sustained-release gel
ActiveCN113018249AGood bioadhesionGood slow releaseOrganic active ingredientsAntimycoticsSodium bicarbonateCellulose
The invention provides an oral sustained-release gel for treating candida mycoderma and a preparation method thereof, belongs to the field of pharmaceutical preparations, and solves the problems that patients are susceptible to serious side effects and the like due to the adoption of a systemic administration mode for a long time in the prior art. The oral sustained-release gel for treating the candida mycoderma is characterized by comprising the following raw materials in parts by volume: 20-30 parts of liposome, 30-45 parts of a gel material, 10-20 parts of a sodium bicarbonate solution with the concentration of 1%-4% and 5-8 parts of a molding material, and miconazole and carboxymethyl cellulose are encapsulated in the liposome. The invention has the advantages of local administration, long acting time and the like.
Owner:ZHEJIANG UNIV
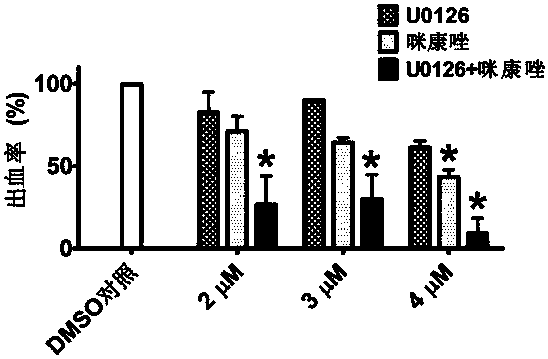
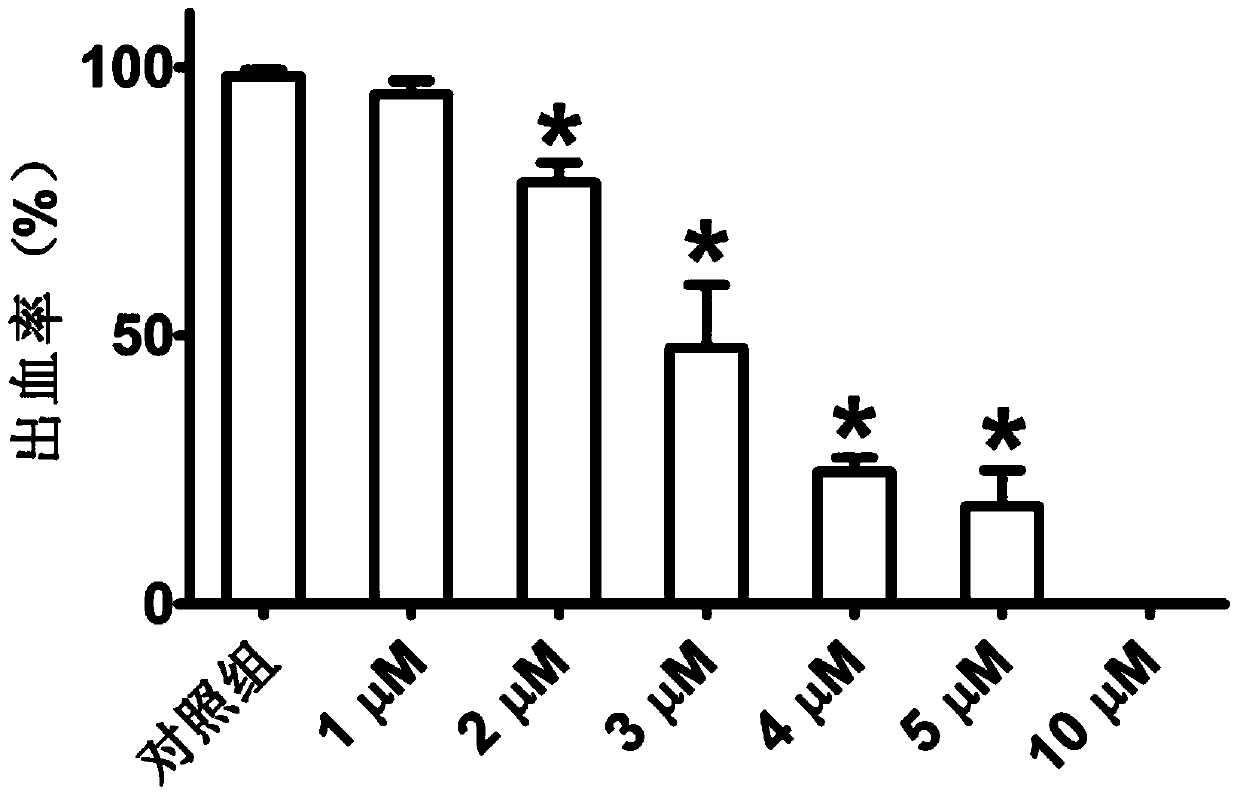
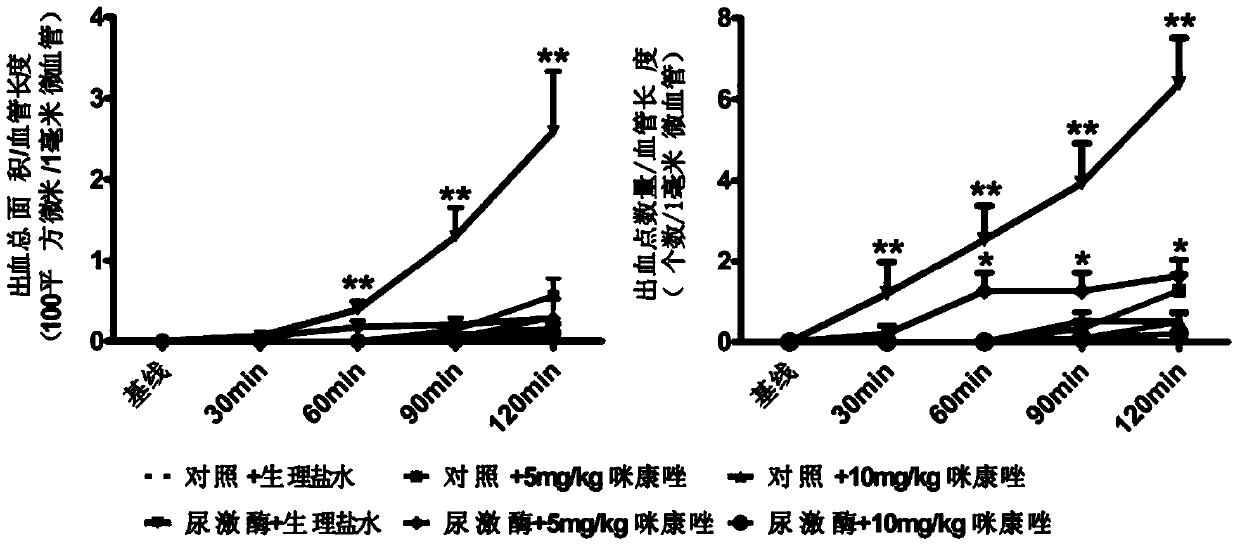
Application of Miconazole in Prevention of Vascular Rupture
ActiveCN107485610BNitrile/isonitrile active ingredientsCardiovascular disorderDiseasePhosphorylation
The invention relates to application of miconazole to preparation of drugs used for preventing vascular rupture. The invention also relates to application of miconazole as a MMP9 inhibitor in vascular peripheral cells. The invention also relates to application of miconazole as an ERK inhibitor in vascular peripheral cells. The invention further relates to a pharmaceutical composition for preventing vascular hemorrhage, wherein the main drug effect component of the pharmaceutical composition is miconazole. The invention also relates to a pharmaceutical composition for inhibiting MMP9 expression in vascular peripheral cells, wherein the main drug effect component of the pharmaceutical composition is miconazole. The invention also relates to a pharmaceutical composition for inhibiting phosphorylation of ERK in vascular peripheral cells, wherein the main drug effect component of the pharmaceutical composition is miconazole. The invention also relates to a pharmaceutical composition which contains U0126 and miconazole. The invention further relates to application of miconazole to prevention of abnormal vascular rupture. The invention also provides novel application of miconazole to prevention of abnormal vascular hemorrhage. Miconazole can effectively prevent a plurality of diseases caused by vascular hemorrhage.
Owner:PEKING UNIV
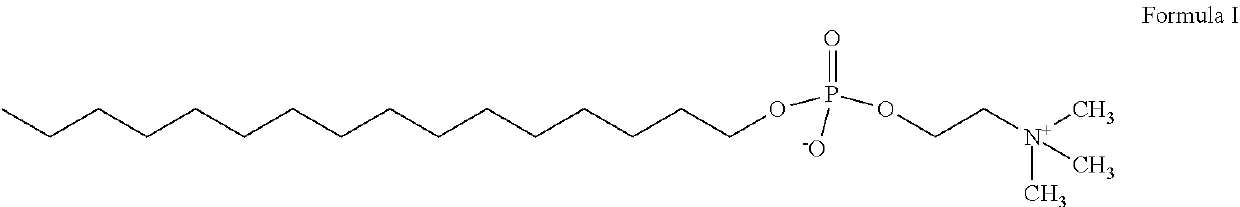
A kind of liquid gel and preparation method thereof
ActiveCN106266037BNo drug resistanceImprove the bactericidal effectAntibacterial agentsAntimycoticsCelluloseDisease
Provided are liquid gel and a preparation method thereof. The liquid gel is characterized in that the gel is prepared from 0.5-2.5 parts by weight of chitosan, 0.5-5 parts by weight of hydroxypropyl methylcellulose, 0.5-6 parts by weight of polyethylene glycol 6000, 0.1-0.3 part by weight of chlorhexidine acetate, 0.1-3 parts by weight of carbomer, 0.1-2 parts by weight of propylene glycol, 0.1-3 parts by weight of Chinese hawthorn seeds, 0.2-2 parts by weight of hyaluronic acid matter, 0.01-0.5 part by weight of miconazole nitrate and 50-70 parts by weight of purified water, and the pH ranges from 5 to 7.5. The liquid gel has the advantages of being small in side effect, good in treatment effect, not likely to cause genital tract flora unbalance and capable of effectively relieving or curing the disease.
Owner:吉安市御美丽健康产业股份有限公司
Western medicine ointment for treating pompholyx
InactiveCN106729605AGood antibacterial effectAntipruriticAntimycoticsPeptide/protein ingredientsGlycerolD ointment
The invention discloses a western medicine ointment for treating pompholyx. The western medicine ointment is prepared from, by weight, 2-5 parts of miconazole nitrate ointments, 2-5 parts of mometasone furoate gel, 5-10 parts of glycerol, 5-8 parts of sea-fish oligopeptide, 0.2-0.5 part of a vitamin E, 2-5 parts of chlorpheniramine maleate, 2-5 parts of amprenavir, 0.01-0.05 part of an anti-inflammation preservative, 1-3 parts of oleanolic acid, 0.1-0.5 part of flavone, 0.5-2 parts of snake oil, 0.01-0.05 part of chondroitin sulfate, 1-2 parts of a terbinafine hydrochloride solution and 10-20 parts of honey. According to the western medicine ointment, the synergistic effect is achieved through raw material compounding, and the western medicine ointment has the effects of being good in antibacterial effect, relieving itching and sterilization, and can effectively treat hand-and-foot moss; the western medicine ointment has the advantages of being not prone to recrudescence, low in medicine dependence, short in treatment cycle and capable of thoroughly and radically treating pompholyx; the western medicine ointment also has the curative effects of activating blood to remove stasis, resisting inflammation, relieving pains and preventing infection.
Owner:HENAN BALING ELECTRONICS TECH CO LTD
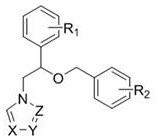
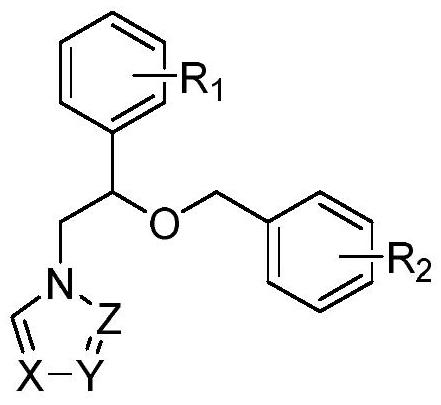
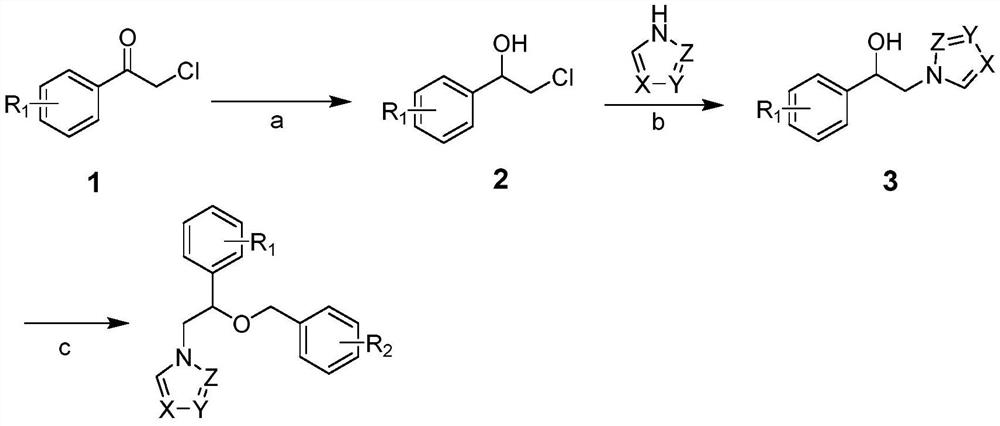
Use of miconazole and its derivatives as Tgr5 agonists
ActiveCN111039880BOvert agonistic activityProminent TGR5 agonistic activityOrganic active ingredientsOrganic chemistryDiseaseMicronazole
The invention belongs to the technical field of medicine, and relates to the new application of miconazole and derivatives thereof in treating diseases and diseases of biopathological processes in which TGR5 participates. The structural formulas of the miconazole and its derivatives are as follows. The miconazole and its analogues can activate TGR5 activity, and can be used for treating or preventing diseases related to the regulation of TGR5 activity.
Owner:HENAN UNIVERSITY
A kind of beriberi preparation and preparation method thereof
The invention discloses a dermatophytosis preparation. In the dermatophytosis preparation, miconazole nitrate and triclosan are main bactericidal components and can generate a synergistic effect by matched use to enhance a bactericidal effect; urotropin mainly plays roles in convergence and hidroschesis in a formula and simultaneously has a bactericidal effect; hexadecadrol is a corticosteroid drug and mainly plays anti-inflammatory and anti-pruritic roles in the formula; dimethyl sulfoxide mainly plays a solubilizing role in the formula to dissolve the miconazole nitrate and the hexadecadroland simultaneously has a trans-dermal effect to enhance the trans-dermal absorption of the drug and enhance the drug effect; laurocapram is a trans-dermal absorbing agent to enhance the drug trans-dermal absorption function; glycerol is a moisturizing agent and mainly plays roles in moisturizing skin, reducing irritation and increasing drug adhesion in the formula; medical ethanol is mainly used as a solvent and simultaneously has a bactericidal effect; menthol and essence are taken as flavoring agents to improve the drug odor and improve the use experience; the dermatophytosis preparation simultaneously has the effect of covering the foot odor.
Owner:KUNMING BEIJIAN BIOTECH
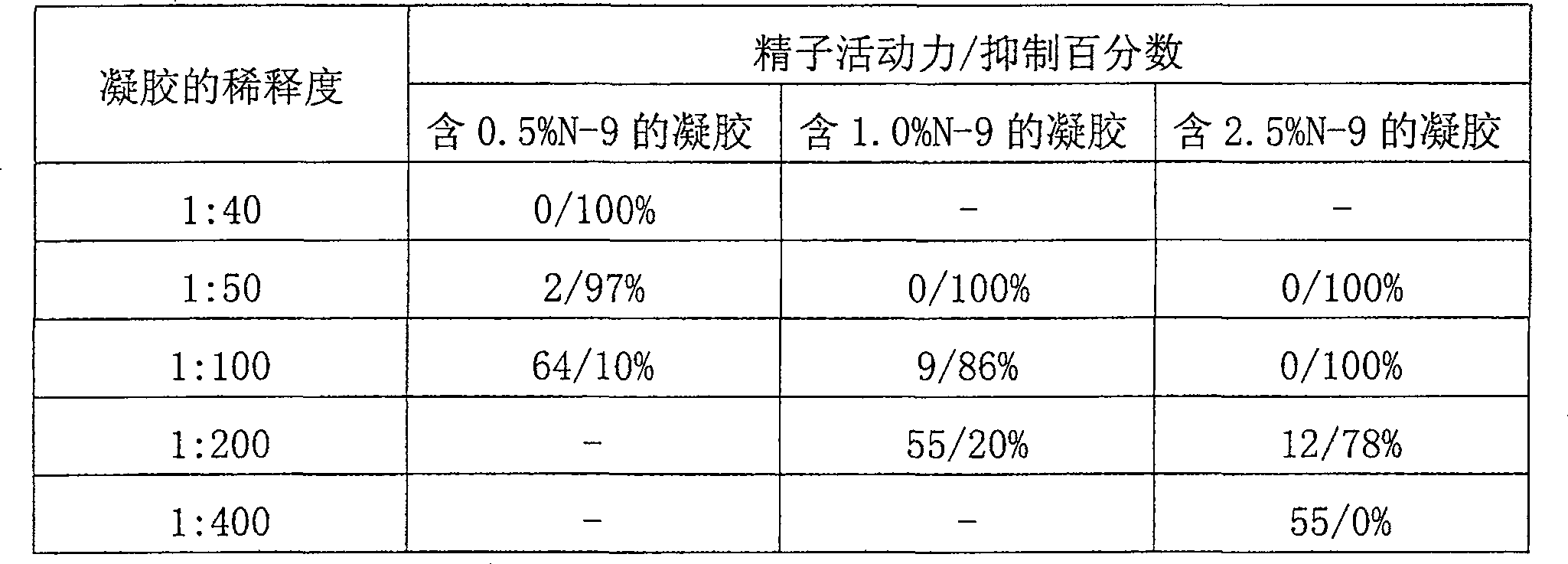
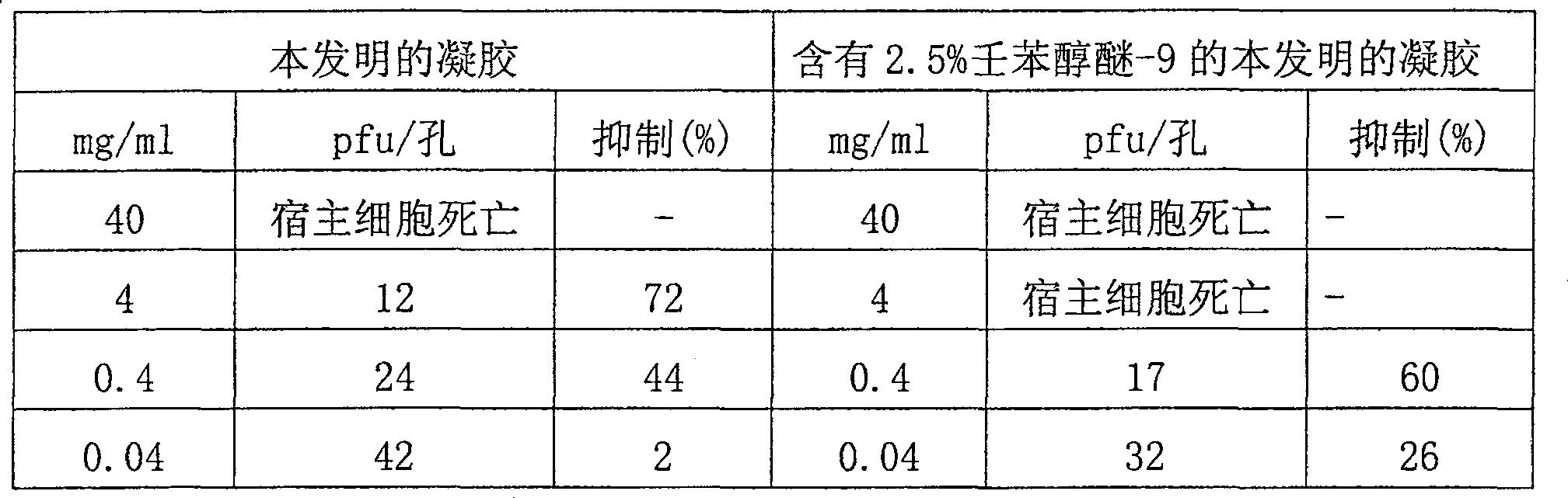
Composition for trapping and inactivating pathogenic microbes and spermatozoa and its pharmaceutical uses
Antimicrobial and contraceptive compositions and methods are provided that prevent and / or reduce sexually transmitted diseases (STDs) transmitted through sexual activity, and prevent and / or reduce the risk of pregnancy. The composition contains (1) a matrix forming agent, (2) a bioadhesive, (3) a buffer, (4) an optional wetting agent, (5) an optional preservative, and (6) water; wherein the composition is suitable for application within the vagina; wherein the composition forms a semi-solid matrix upon contact with semen (thereby capturing microorganisms and sperm released by ejaculation); wherein the composition causes hardening of cervical mucus (thereby reducing the likelihood of sperm entry); wherein The composition forms a bioadhesive layer on the vaginal surface (thereby preventing or reducing the risk of STD-causing microorganisms coming into contact with the vaginal surface); wherein the composition maintains an acidic vaginal pH of less than about 5 in the presence of semen released by male ejaculation; and wherein the combination The substance does not significantly damage the normal microbial balance in the vagina. Antimicrobial and contraceptive compositions may also contain other antimicrobial and / or contraceptive agents (e.g., nonoxynol-9, octoxynol-9, benzalkonium chloride, phosphorylated hesperidin, sulfonated orange Dermatosine, polystyrene sulfonate, substituted benzenesulfonate formaldehyde copolymer, H 2 SO 4 -Modified mandelic acid, povidone-iodine, itraconazole, ketoconazole, metronidazole, clotrimazole, fluconazole, teraconazole, miconazole, tinidazole, itonazole, chloride (mycin, nystatin, ciclopiroxamide, etc.).
Owner:RUSH UNIV MEDICAL CENT
Tinidazole/miconazole nitrate/neomycin compound vaginal suppository and preparation method thereof
InactiveCN101703516BMedication convenienceIncrease concentrationAntibacterial agentsOrganic active ingredientsMicronazolePharmaceutical formulation
The invention belongs to the field of pharmaceutic preparation and relates to a tinidazole / miconazole nitrate / neomycin compound vaginal suppository and a preparation method thereof. The vaginal suppository consists of tinidazole, miconazole nitrate, neomycin sulfate, suppository substrate and an acidifier with curative doses, and can be used for multiple kinds of colpitis. The preparation method comprises the following steps: heating and smelting a suppository substrate and an acidifier with prescription doses; adding fine powders of the tinidazole, the miconazole nitrate and the neomycin sulfate filtered by a 80-mesh sieve into the mixture; quickly stirring the mixture till the mixture is about to be solidified; filling the mixture into a suppository film; and solidifying, scraping, taking out and packaging or directly filling the mixture into a dual-aluminum foil suppository bag. The prepared tinidazole / miconazole nitrate / neomycin compound vaginal suppository has scientific and novel prescription, is directly acted on a pathological part, has high medicament concentration on local parts, overcomes the gastrointestinal upset caused by oral administration, is convenient for patients to use, improves the treatment effect and reduces the toxicity and side effect. The preparation process is simple, has no special requirement on preparation environment, has low cost and stable quality of products and is suitable for industrialized mass production.
Owner:SHENYANG PHARMA UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com