Microbial limit detection method of miconazole nitrate suppository
A miconazole nitrate suppository and microbiological limit technology, applied in the field of medicine, can solve the problems of slow lipid-water layering, destroying the living environment of bacteria, prolonging the test time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
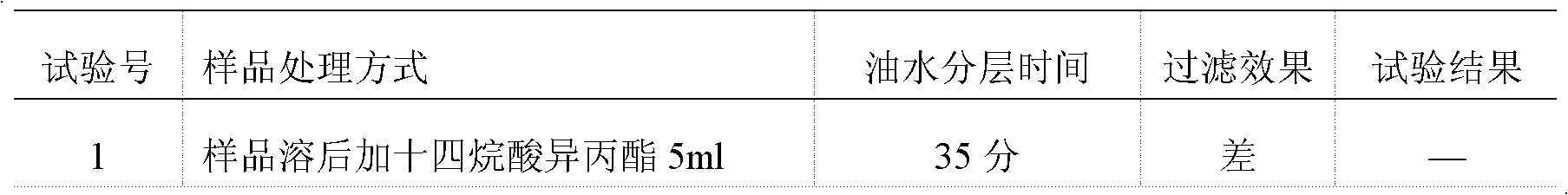
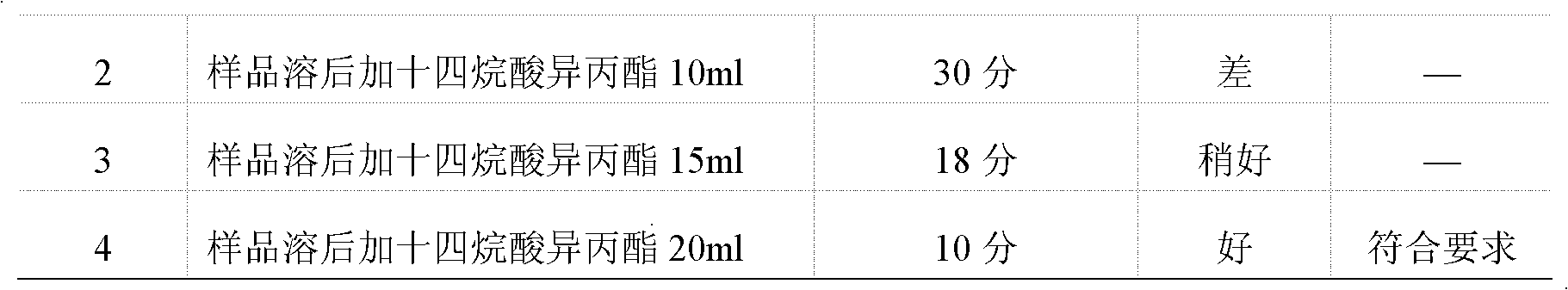
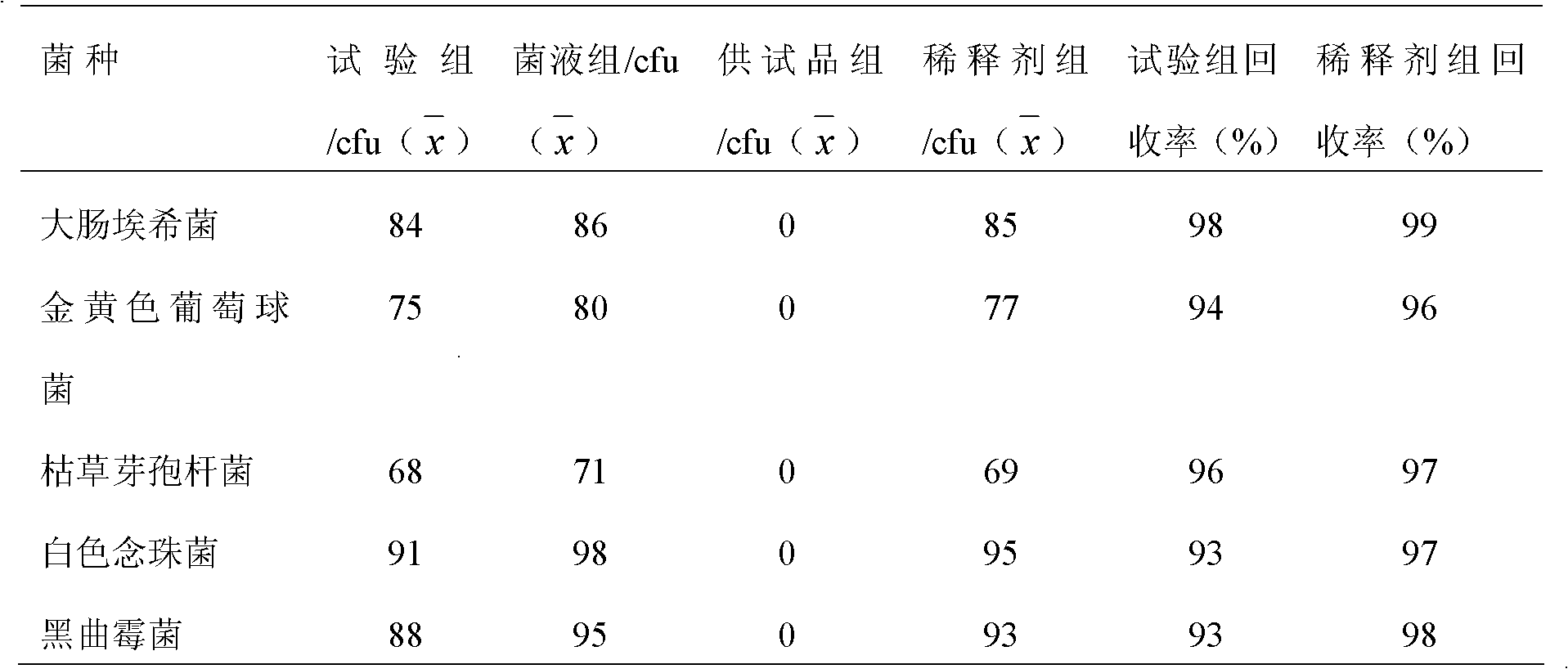
[0049] Concrete test process of the present invention is as follows:
[0050] 1 Instruments and materials
[0051] 1.1 Instrument
[0052] Water-proof electric heating constant temperature incubator Chongqing Four Experimental Instrument Factory production model: WGP-600
[0053] Biochemical incubator Shanghai Yiheng Technology Co., Ltd. production model: LRH-250
[0054] Mold incubator Chongqing Wanda production model: SHH-250JS
[0055] Biological safety cabinet Shanghai Lishen Scientific Instrument Co., Ltd. Production model: HF Safe 760s
[0056] 1.2 Medium
[0057] Nutrient agar medium batch number: 090912 produced by Beijing Sanyao Science and Technology Development Company
[0058] Rose Bengal Sodium Agar Medium Lot Number: 0911172 Produced by Beijing Sanyao Technology Development Company
[0059] Nutrient broth culture medium Batch number: 100310, produced by Beijing Sanyao Science and Technology Development Company
[0060] Improved Martin Medium Batch No.: 091...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com