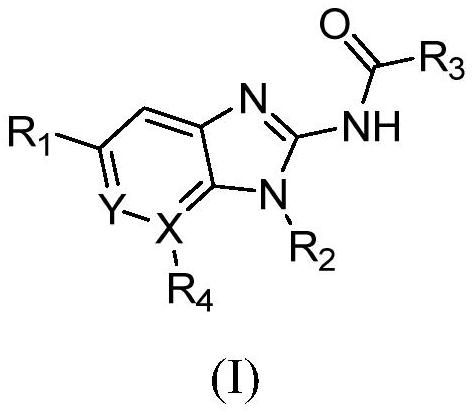
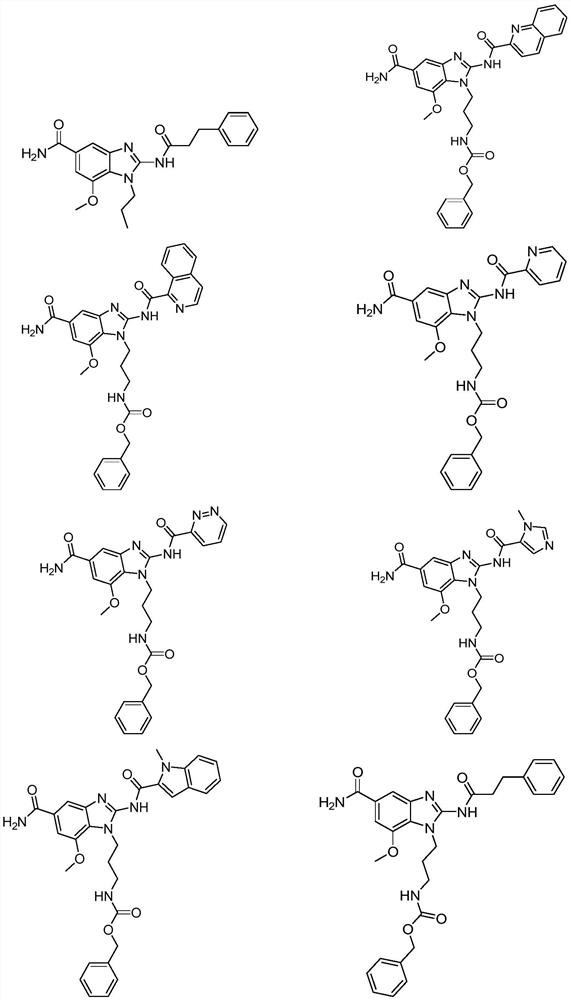
Aromatic ring or aromatic heterocyclic imidazole compound, its preparation method and pharmaceutical use
A compound and pharmaceutical technology, applied in drug combination, organic chemistry, antineoplastic drugs, etc., can solve problems such as changes in the hematopoietic system, instability of natural ligand molecules, cyclic dinucleosides, and poor druggability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
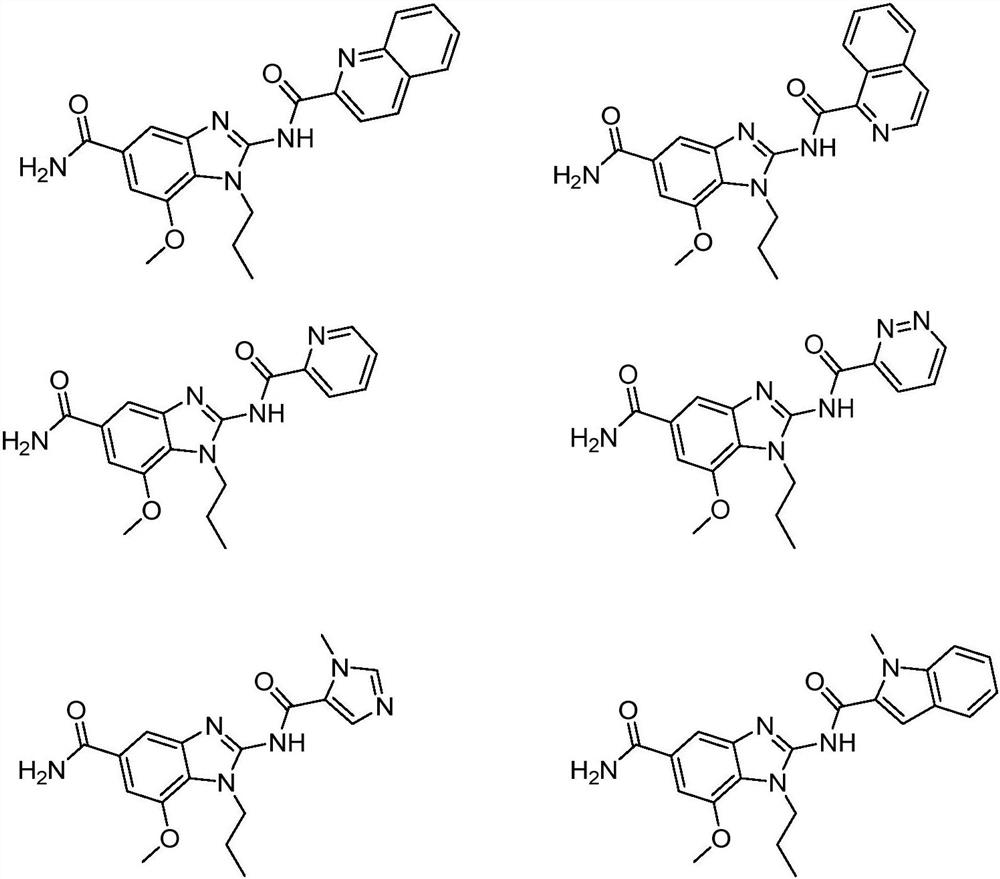
[0046] N-(5-carbamoyl-7-methoxy-1-propyl-1H-benzo[d]imidazol-2-yl)quinoline-2-amide
[0047]
[0048] (1) Preparation of 4-chloro-3-methoxy-5-nitrobenzamide
[0049]
[0050] Methyl 3-methoxy-4-propylamino-5-nitrobenzenecarboxylate (1 g, 4.1 mmol), 50 ml of ammonia water (28%, 50 ml) were added to the bottle in turn, and heated to 50 ° C under argon protection for the reaction 5h. The reaction was cooled to room temperature, filtered under reduced pressure after a large amount of light yellow solid was precipitated, and the filter cake was washed with ether and dried to obtain 0.715 g of light yellow solid, yield 75.6%, melting point 215-217°C.
[0051] 1 H NMR (400MHz, DMSO-d 6 )δ8.29(s,1H),8.05(d,J=1.64Hz,1H),7.88(d,J=1.56Hz,1H),7.78(s,1H),4.02(s,3H).
[0052] MS(ESI) m / z 231.0(M+H) + .
[0053] (2) Preparation of 3-methoxy-5-nitro-4-propylaminobenzamide
[0054]
[0055] 4-Chloro-3-methoxy-5-nitrobenzamide (1 g, 4 mmol), propylamine (0.715 ml, 8.7 mmol), pot...
Embodiment 2
[0072] N-(5-carbamoyl-7-methoxy-1-propyl-1H-benzo[d]imidazol-2-yl)isoquinoline-1-amide
[0073]
[0074] (1) Preparation of the title compound
[0075] 2-Amino-7-methoxy-1-propyl-1H-benzo[d]imidazole-5-amide (0.2 g, 0.81 mmol), isoquinoline-1-carboxylic acid (0.168 g, 0.97 mmol) ), 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethylurea hexafluorophosphate (0.368g, 0.97mmol), N,N-diisopropylethyl acetate The amine (0.54ml, 3.25mmol) was dissolved in 10ml N,N-dimethylformamide, and the reaction was stirred at room temperature for 5h. After the reaction, 30 ml of saturated ammonium chloride solution was added, and a large amount of light orange solid was precipitated, which was filtered under reduced pressure, and the filter cake was washed with ether and dried to obtain 0.149 g of light orange solid, yield 45.5%, melting point 251-252 ° C.
[0076] 1 H NMR (400MHz, DMSO-d 6 )δ8.57–8.51(m,1H),8.37(d,J=5.7Hz,1H),7.89(d,J=8.2Hz,1H),7.74(d,J=1.2Hz,1H),7.70– 7.66(m, 2H), 7.54(t,...
Embodiment 3
[0079] 7-Methoxy-2-(2-pyridylamido)-1-propyl-1H-benzo[d]imidazole-5-amide
[0080]
[0081] (1) Preparation of the title compound
[0082]2-Amino-7-methoxy-1-propyl-1H-benzo[d]imidazole-5-amide (0.2 g, 0.81 mmol), pyridine-2-carboxylic acid (0.120 g, 0.97 mmol), 2-(7-Benzotriazole oxide)-N,N,N',N'-tetramethylurea hexafluorophosphate (0.368g, 0.97mmol), N,N-diisopropylethylamine ( 0.54ml, 3.25mmol) was dissolved in 10ml N,N-dimethylformamide, and the reaction was stirred at room temperature for 8h. After the reaction, 30 ml of saturated ammonium chloride solution was added, and a large amount of white solid was precipitated, which was filtered under reduced pressure, the filter cake was washed with ether, and dried to obtain 183 mg of white solid, yield 64.1%, melting point>300°C.
[0083] 1 H NMR (400MHz, DMSO-d 6 )δ8.65(d,J=4.0Hz,1H),8.40(d,J=7.8Hz,1H),7.88(t,J=7.8Hz,1H),7.83(s,2H),7.50–7.37( m, 1H), 7.19(s, 1H), 7.13(s, 1H), 4.36(t, J=6.7Hz, 2H), 3.94(s, 3H), 1.80–1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com