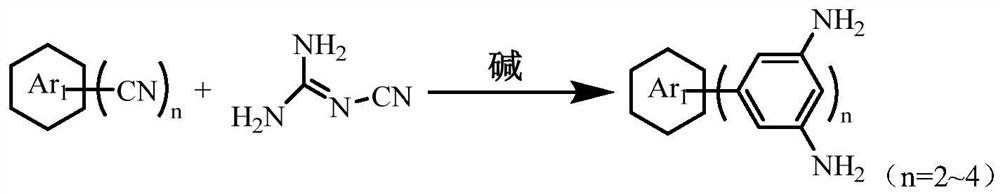
Nitrogen-rich porous polymer containing heteroatom, imine and triazine ring structure and preparation method
A technology of porous polymers and triazine rings, applied in the field of polymer science, can solve unseen problems and achieve the effects of improving adsorption and separation efficiency, good application prospects, and high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
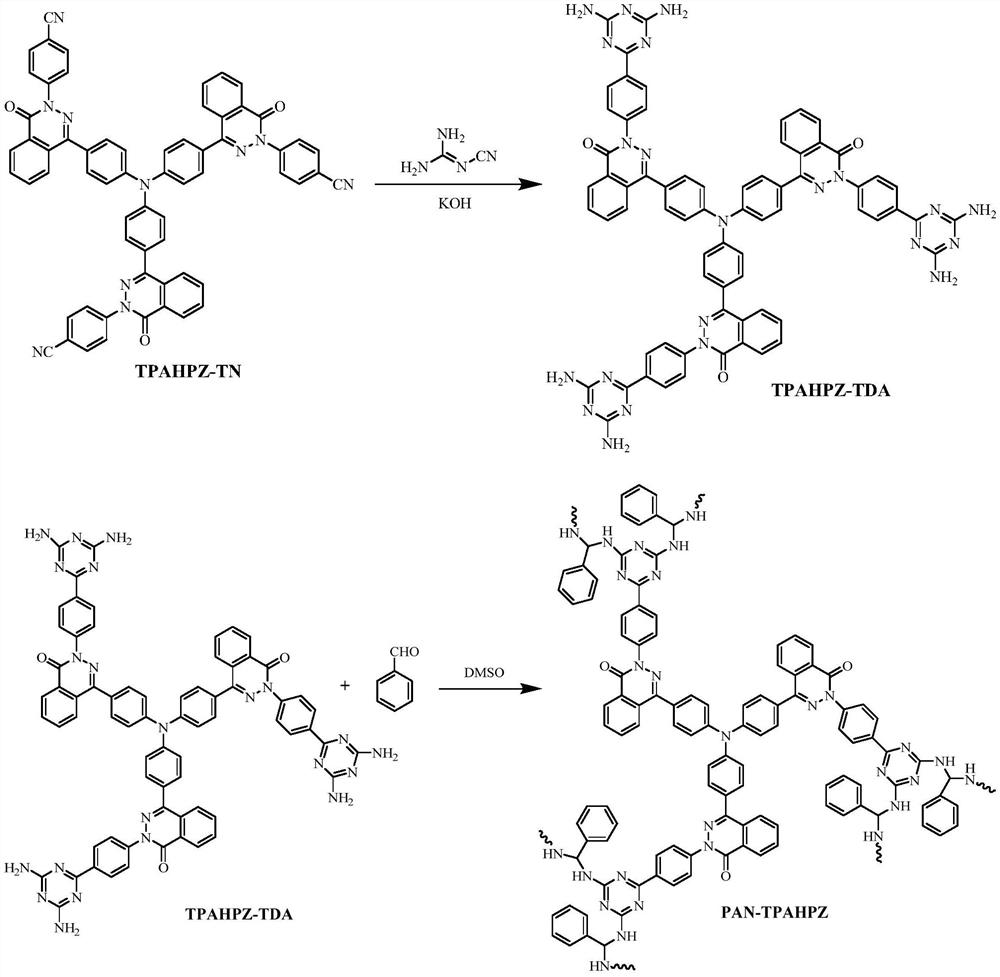
[0031] Example 1, preparation of nitrogen-rich porous polymer PAN-TPAHPZ containing triazine ring, phthalazinone biphenyl and imine structure
[0032] Tris[4-(4-(2-(4-cyanophenyl))-2,3-naphthyridine-1-one)phenyl]amine (TPAHPZ-TN) (2mmol), dicyandiamide (12mmol), KOH (4mmol) and ethylene glycol methyl ether (2.8mL) were mixed uniformly in a three-necked flask, and then the temperature was gradually raised to 160° C. for 1 h under nitrogen atmosphere. Submerge the suspension obtained after the reaction into hot water, filter out the solid product, wash with hot water, and dry to obtain TPAHPZ-TDA, a polyamine monomer containing a triazine ring and a phthalazinone biphenyl structure.
[0033] Add polyamine monomer TPAHPZ-TDA (0.4mmol), benzaldehyde (2.4mmol) and dimethyl sulfoxide (DMSO, 2.8mL) into the nitrogen-substituted reaction flask, and then replace nitrogen three times. The reactant was slowly warmed up to 100°C and reacted under DMSO reflux for 100h. Afterwards, the mi...
Embodiment 2
[0036] Example 2, preparation of porous polymer PAN-POTDA containing pyridine, triazine ring, thiophene and imine structure
[0037] Weigh pyridine 2,6-dinitrile (2mmol), dicyandiamide (8mmol), KOH (2.2mmol) and ethylene glycol diethyl ether (200mL), add them into a three-necked flask and mix them evenly. Gradually raise the temperature to 80°C and react for 24h. The suspension obtained after the reaction is poured into hot water, and the product solid is filtered out, washed with hot water, and dried to obtain the polyamine monomer POTDA containing pyridine and triazine ring structures.
[0038]Add polyamine monomer POTDA (2.0 mmol), 2-thiophenecarbaldehyde (8.0 mmol) and N-methylpyrrolidone (NMP, 200 mL) into the nitrogen-substituted reaction flask, and then replace nitrogen three times. The reactants were slowly warmed up to 210°C for 10 hours. Afterwards, the mixture was suction filtered and washed with N,N-dimethylformamide and methanol respectively to obtain a solid, a...
Embodiment 3
[0041] Embodiment 3, the preparation that contains pyridine ring, triazine ring and imine structure porous polymer PAN-POTDA
[0042] Weigh pyridine 2,6-dinitrile (2mmol), dicyandiamide (20mmol), KOH (20mmol) and ethylene glycol diethyl ether (200mL), add them into a three-necked flask and mix well, gradually Raise the temperature to 150°C and react for 10h. The suspension obtained after the reaction is poured into hot water, and the product solid is filtered out, washed with hot water, and dried to obtain a polyamine monomer POTDA containing a pyridine ring and a triazine ring structure.
[0043] Add polyamine monomer POTDA (2.0 mmol), terephthalaldehyde (8.0 mmol) and dimethyl sulfoxide (DMSO, 60 mL) into the nitrogen-substituted reaction flask, and then replace nitrogen three times. The reactant was slowly warmed up to 180°C for 50h. Afterwards, the mixture was suction-filtered and washed with N,N-dimethylformamide and methanol respectively to obtain a solid, and then the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com