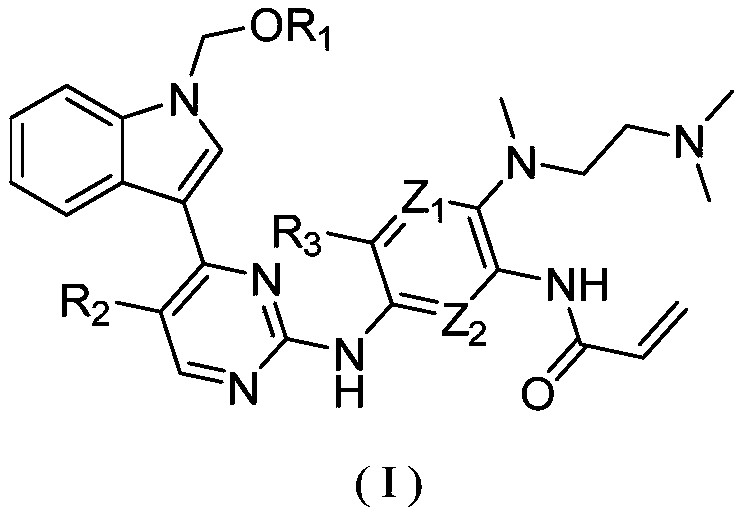
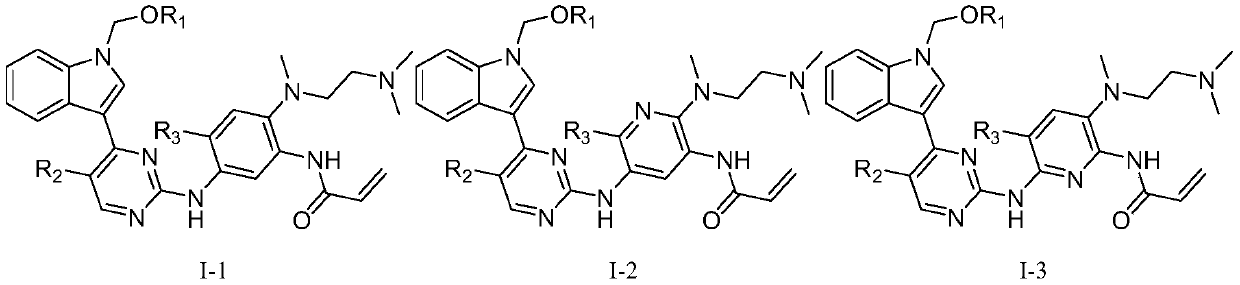
2-(2,4,5-substituted anilino)pyrimidine compound and application thereof
A compound, C1-C4 technology, applied in 2-(2,4,5-substituted anilino)pyrimidine compounds and its application field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
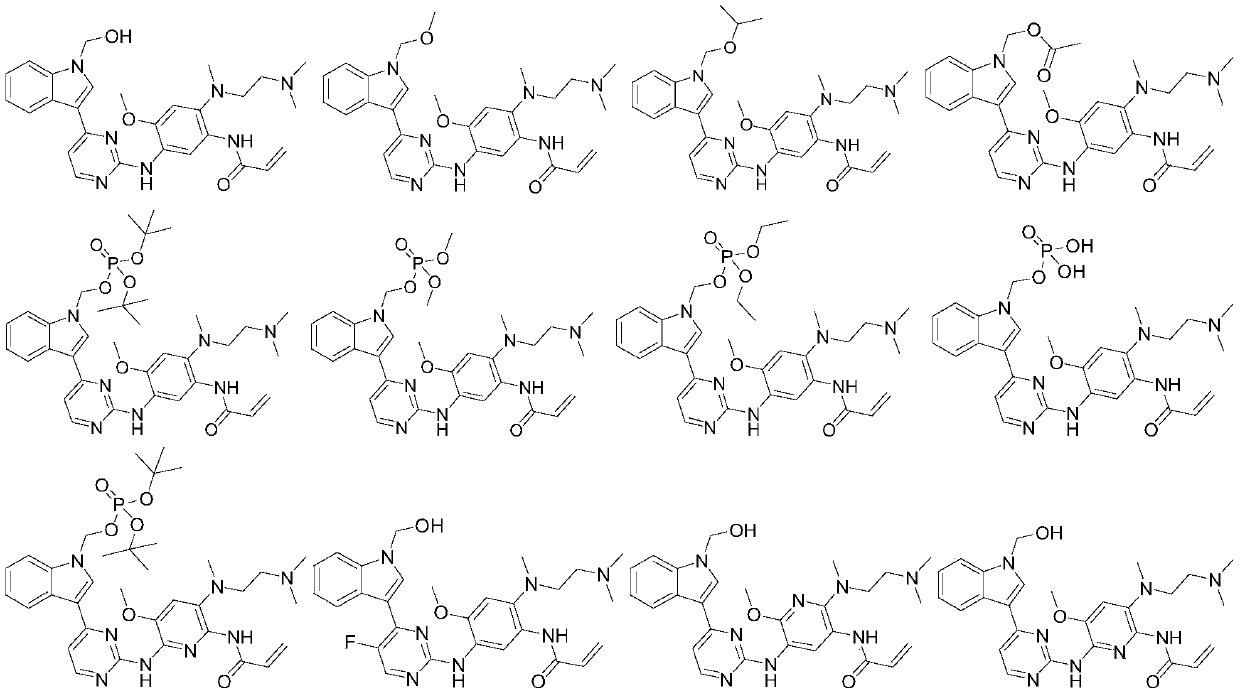
[0105] Example 1: N-(2-{2-dimethylaminoethyl-methylamino}-4-methoxy-5-{[4-(1-hydroxymethyl-indol-3-yl)pyrimidine- 2-yl]amino}phenyl)prop-2-enamide
[0106]
[0107] Step a: Preparation of 3-(2-chloro-pyrimidin-4-yl)-1H-indole (intermediate 1)
[0108] Dissolve indole (10.0g, 85mmol) in 1,2-dichloroethane (100mL), slowly add methylmagnesium bromide (3M, 28.5mL) dropwise at 0°C, after dropping, stir under ice bath After 15 minutes, 2,4-dichloropyrimidine (19.1 g, 128 mmol) was added to the above reaction solution all at once, and stirred overnight at room temperature. With stirring at room temperature, the above reaction solution was added dropwise to 1M dilute hydrochloric acid, a solid was precipitated, filtered with suction, and dried to obtain 11.0 g, with a yield of 56.3%.
[0109] Step b: Preparation of N-(4-fluoro-2-methoxy-5-nitrophenyl)-4-(indol-3-yl)pyrimidin-2-amine (intermediate 2)
[0110] Dissolve Intermediate 1 (11.0g, 48mmol), 4-fluoro-2-methoxy-5-nitroanil...
Embodiment 1A
[0124] Example 1A: N-(2-{2-Dimethylaminoethyl-methylamino}-4-methoxy-5-{[4-(1-hydroxymethyl-indol-3-yl)pyrimidine- 2-yl]amino}phenyl)prop-2-enamide methanesulfonate
[0125]
[0126] At 70°C, to N-(2-{2-dimethylaminoethyl-methylamino}-4-methoxy-5-{[4-(1-hydroxymethyl-indol-3-yl) Pyrimidin-2-yl]amino}phenyl)prop-2-enamide (500mg, 0.97mmol) in a mixed solvent of ethanol (10mL) and EtOAc (8mL) was slowly added dropwise methanesulfonic acid (93mg, 0.97mmol) solution in EtOAc (4 mL). Keep stirring for 1.5 hours. Filtrate while hot, and dry in vacuo at 80° C. to obtain 530 mg of a light yellow solid with a yield of 89.3%.
[0127] ESI-MS m / z:516.4[M+H] + .
[0128] 1 H NMR (400MHz, DMSO-d 6 )δ:9.55(s,1H),9.26(s,1H),8.70(s,1H),8.56(s,1H),8.31(s,1H),7.67(d,J=4.0Hz,1H), 7.35(d, J=4.0Hz, 1H), 7.27(t, J=8.0Hz, 1H), 7.16(t, J=8.0Hz, 1H), 7.05(s, 1H), 6.73-6.66(m, 1H ),6.35-6.30(m,1H),5.82-5.79(m,1H),5.63(s,2H),3.88(s,3H),3.39-3.11(m,4H),2.82(d,J=4.0 Hz,6H),2.65(s,3H),2.32(s,3...
Embodiment 2
[0129] Example 2: N-(2-{2-dimethylaminoethyl-methylamino}-4-methoxy-5-{[4-(1-methoxymethyl-indol-3-yl) pyrimidin-2-yl]amino}phenyl)prop-2-enamide
[0130]
[0131] N-(2-{2-dimethylaminoethyl-methylamino}-4-methoxy-5-{[4-(1-hydroxymethyl-indol-3-yl)pyrimidin-2-yl ]Amino}phenyl)prop-2-enamide (100mg, 0.194mmol) was dissolved in 2mL of dry DMF, NaH (9.3mg, 0.233mmol) was added under ice-cooling, after the addition was completed, it was reacted at 0°C for 0.5h, and then Iodomethane (33mg, 0.233mmol) was dissolved in DMF and added to the reaction solution, reacted at room temperature for 2h, the reaction solution was poured into water, extracted with DCM, the organic phase was washed with saturated sodium chloride, dried over anhydrous sodium sulfate, and column purified 25 mg of solid was obtained, yield: 24.3%.
[0132] ESI-MS m / z:530.4[M+H] + .
[0133] 1 H NMR (400MHz, CDCl 3)δ:10.49(s,1H),9.65(s,1H),9.29(s,1H),8.40(d,J=4.0Hz,1H),7.98(d,J=8.0Hz,1H),7.79( s,1H),7.55(d,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com