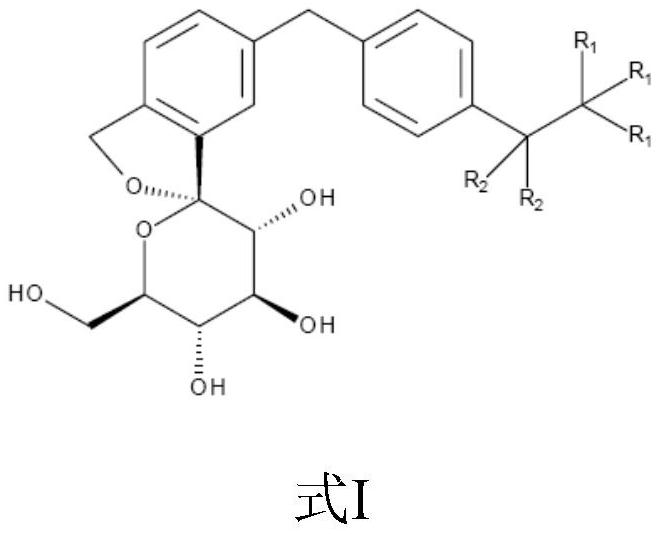
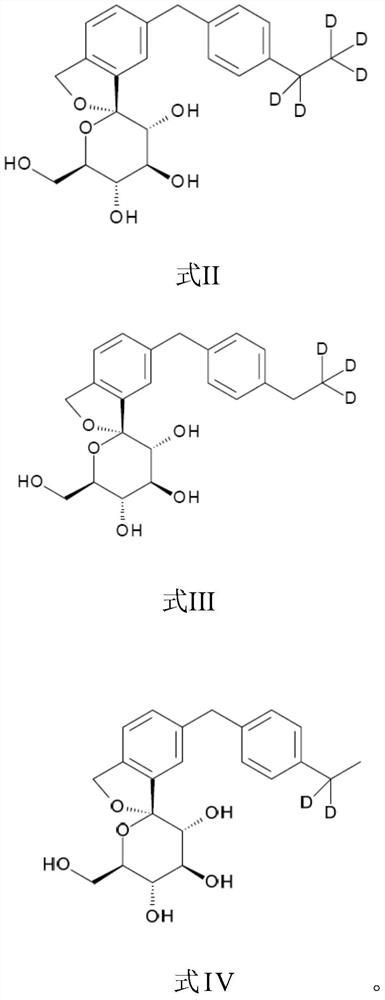
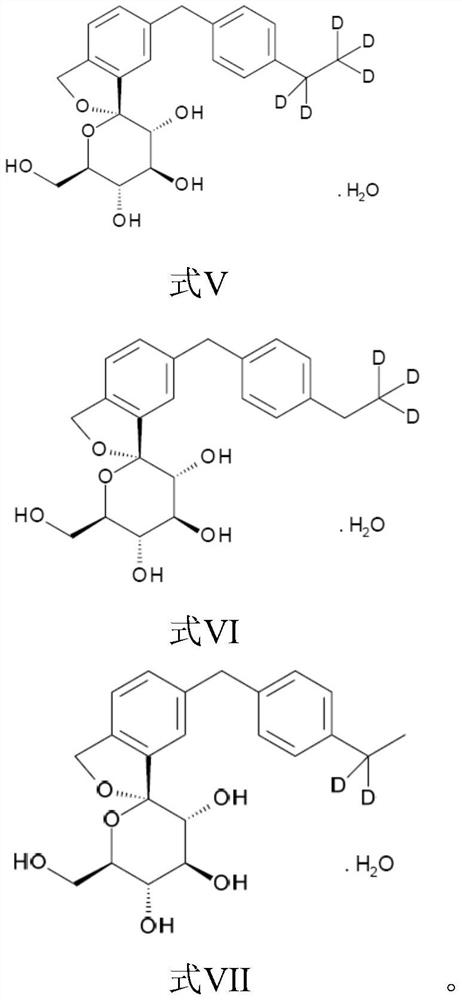
Isobenzofuran derivatives, pharmaceutical compositions and preparations thereof, and uses thereof
A technology of derivatives and compositions, applied in the field of medicine, can solve the problems of poor selectivity of SGLT1 and SGLT2, large adverse reactions, and failure to apply on a large scale, and achieve low toxicity, excellent pharmacodynamic properties, and good SGLT-2 inhibition active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[0076] (1S, 3'R, 4'S, 5'S, 6'R)-6-[(4-pentadeuterioethylphenyl)methyl]-3', 4', 5',6'-tetrahydro-6'-(hydroxymethyl)spiro[isobenzofuran-1(3H),2'-[2H]pyran]-3',4',5'-triol
[0077]
[0078]
[0079] Step 1: Preparation of intermediate (1S, 3'R, 4'S, 5'S, 6'R)-6-[(methoxycarbonyloxy)methyl]-3',4',5',6'-tetrahydro -3',4',5'-bis(methoxycarbonyloxy)-6'-[(methoxycarbonyloxy)methyl]-spiro[isobenzofuran-I(3H),2'-[ 2H]pyran](compound 2)
[0080] Under argon, the commercial product (1S,3'R,4'S,5'S,6'R)-tetrahydro-6,6'-bis(hydroxymethyl)-spiro[isobenzofuran-I(3H), 2'-[2H]pyran]-3',4',5'-triol (compound 1) (1 equivalent) and 4-dimethylaminopyridine (10 equivalents) were dissolved in anhydrous acetonitrile, ice bath Under cooling, methyl chloroformate (7 eq.) was added slowly. After the addition was complete, the mixture was warmed to 25°C and stirred for 1 hour. LC-MS and TLC showed that the reaction of the intermediate was basically complete. Water was added to terminate the re...
Embodiment 1a-2
[0088] (1S, 3'R, 4'S, 5'S, 6'R)-6-[(4-pentadeuterioethylphenyl)methyl]-3', 4', 5',6'-tetrahydro-6'-(hydroxymethyl)spiro[isobenzofuran-1(3H),2'-[2H]pyran]-3',4',5'-triol .
[0089]
[0090] Step 1: Preparation of Compound 7
[0091] With stirring at room temperature, to Ac 2 Sodium periodate (2.8g, 12.9mmol, 0.56eq) and iodine (2.2g, 8.7mmol, 0.37eq) were sequentially added to a mixed solution of O (9mL) and acetic acid (18mL). After addition, cool to 0°C in an ice-salt bath. Concentrated sulfuric acid (17.7g, 180.4mmol, 7.8eq) was added dropwise to the above mixture. Control the internal temperature not higher than 5°C. After dropping, stir for 10 minutes. Then compound 6 (4.5g, 23.2mmol, 1.0eq) was added in batches, and the addition was completed within 30 minutes, and the internal temperature was controlled not to be higher than 0°C. After the addition, the temperature was raised to room temperature and stirred for 1 hour, then the temperature was raised to 30-45° ...
Embodiment 1b
[0107] The compound shown in preparation formula V
[0108] Under the protection of argon, the compound represented by formula II was dissolved in acetone and water (volume ratio 1:1.5, 300% by weight), cooled to 5°C, and water (100% by weight) was added to the mixture to crystallize 24 hours. The resulting crystals were centrifuged to obtain the compound (Formula V) as monohydrate crystals (moisture content: 4.40% by weight). LC-MS: 392 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com