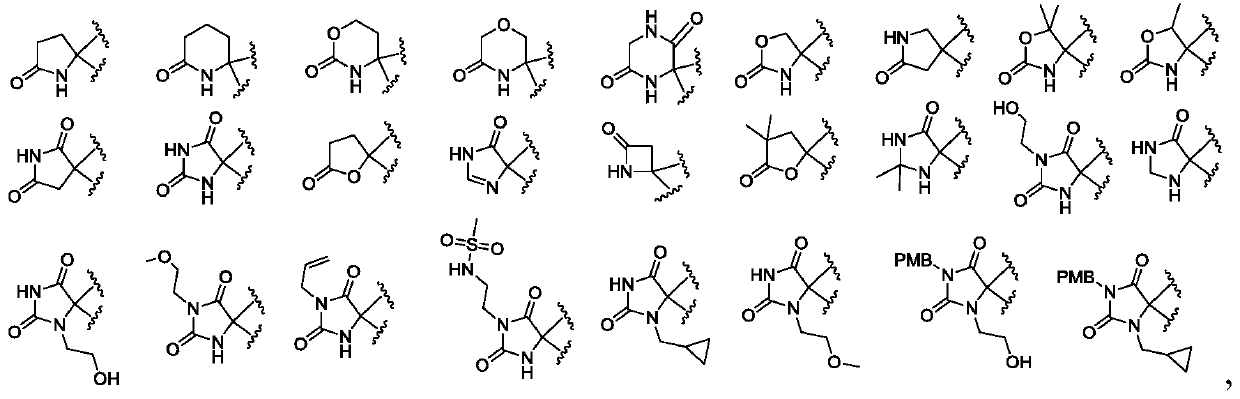
Substituted heterocyclic compound and derivative thereof, pharmaceutical composition, preparation method and use thereof
A compound and derivative technology, applied in the field of medicine, can solve problems such as insufficient activity and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
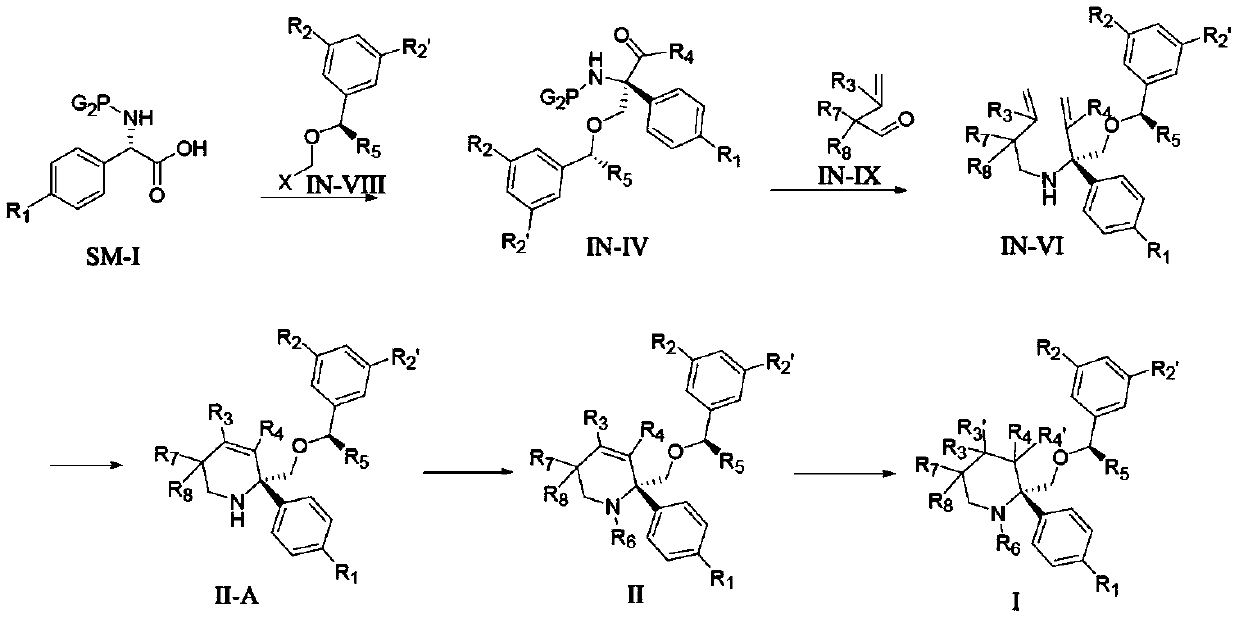
preparation example Construction
[0154] The preparation of solvates is generally known. Typical, non-limiting procedures include dissolving the compound in the required amount of the desired solvent (organic or water or mixtures thereof) at above ambient temperature, cooling the solution at a rate sufficient to form crystals, and then isolating the crystals by standard methods. Analytical techniques such as infrared spectroscopy can confirm the presence of solvent (or water) in crystals as solvates (or hydrates).
[0155] The term "prodrug" as used herein means a substance that is transformed in vivo into a compound having formula (A) or the structure of formula (A) or a pharmaceutically acceptable salt of the compound. Transformation can be accomplished by various mechanisms (eg, by metabolic or chemical processing), eg, by hydrolysis in blood.
[0156] The compounds described herein can be administered alone or in combination with other pharmaceutically acceptable compounds.
[0157] As used herein isomer...
Embodiment 1
[0241] (5S,8S,9R,10s)-8-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)ethoxy)methyl)-9,10-dideuterium- Preparation of 8-phenyl-1,7-diazaspiro[4.5]-2-decanone (I-1)
[0242] Compound A1 (1.0 g, 2.0 mmol) was added to 20 mL of CD at room temperature 3 In OD, add 100 mg of 10% Pd / C, shake well, replace with deuterium and stir at room temperature until the reaction is complete. After filtration, the filtrate was concentrated and the residue was separated and purified by column chromatography to obtain compound I-1 (about 970 mg, yield 96.2%).
[0243] 1 HNMR (400MHz, CDCl 3 )δ:7.88(br,1H),7.61(s,1H),7.41-7.29(m,5H),7.19(s,2H),4.37(q,1H,J=6.4Hz),3.80-3.78(m ,1H),3.20-3.18(m,2H),2.79-2.76(m,1H),2.35-2.30(m,2H),2.05-2.03(m,1H),1.80-1.61(m,3H),1.33 (d,3H,J=6.4Hz).
[0244] MS m / z(ESI):503.1[M+H] +
[0245] The reaction route is:
[0246]
Embodiment 2
[0248] (5R,8S)-8-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)ethoxy)methyl)-8-(4-fluorophenyl)-1 , Preparation of 7-diazaspiro[4.5]-9-double bond-2-decanone (II-1)
[0249] Step 1, the preparation of (S)-2-((((benzyloxy)carbonyl)amino)-2-(4-fluorophenyl)acetic acid (1a)
[0250] Sodium hydroxide (38 g, 0.95 mol) was dissolved in 500 mL of H at room temperature 2 In O, compound SM-1 (70 g, 0.42 mol) was added. Under cooling in an ice bath, 98% Cbz-Cl (68 mL, 0.50 mol) was added to the reaction liquid, and stirred at room temperature until the reaction was complete. Then 6N HCl was added to the reaction solution to adjust the pH to 1-2, filtered, the filter cake was washed with water until the filtrate was neutral, and vacuum-dried at 55° C. for 20 h to obtain the target product 1a (96 g, yield 75.4%).
[0251] MS m / z(ESI):302.2[M+H] +
[0252] Step 2, Preparation of (2R,4S)-benzyl 4-(4-fluorophenyl)-5-carbonyl-2-phenyloxazolidine-3-carboxylate (1b)
[0253] At room temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com