18F-labeled isoquinolinopyridazinone compound as well as synthesis method and application thereof
A synthesis method and technology of isoquinoline, which are applied in the directions of organic chemistry methods, chemical instruments and methods, isotope introduction of heterocyclic compounds, etc., can solve problems such as application to be studied, and achieve the effect of high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
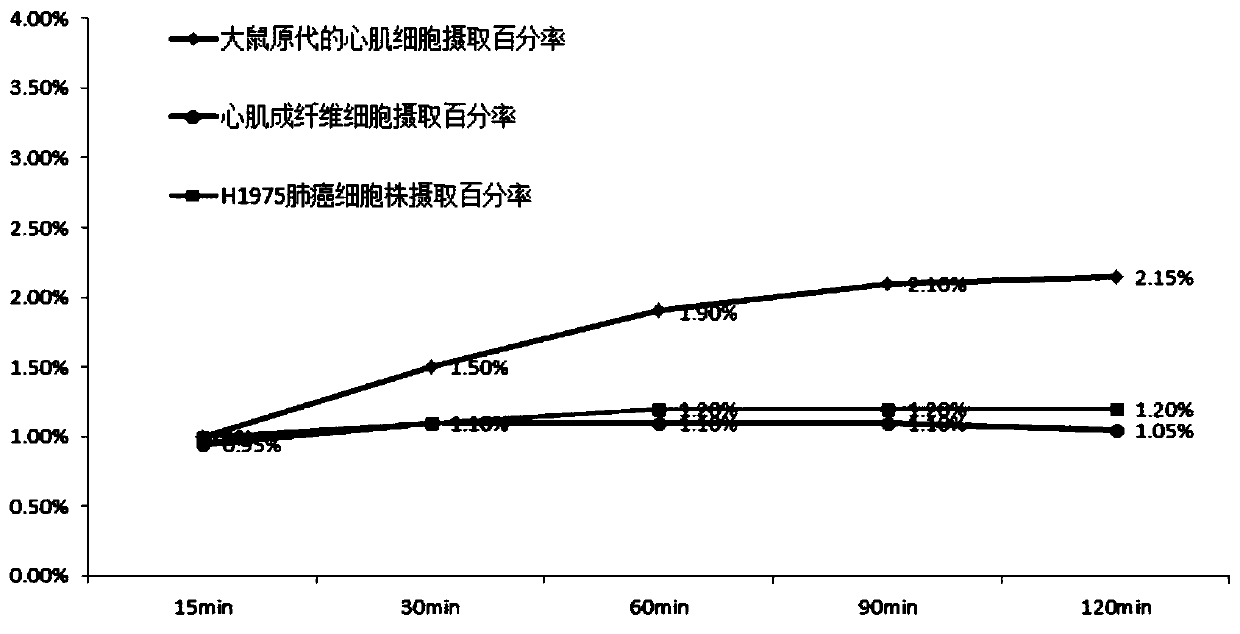
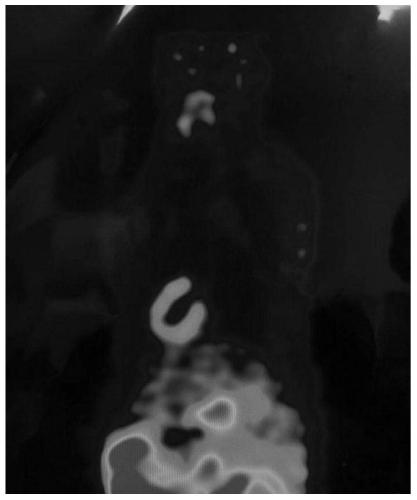
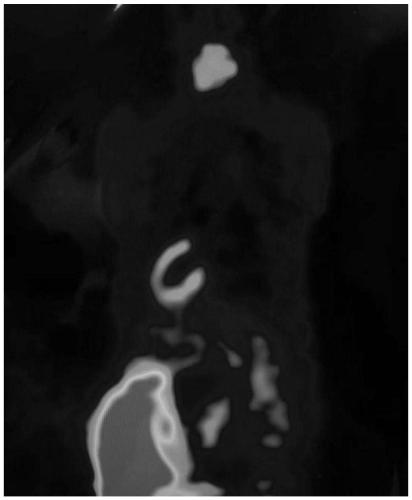
[0033] In order to further illustrate the technical effect of the present invention, the present invention is specifically described below through examples.
[0034] 18 The specific preparation method of the isoquinolinopyridazinone compound of F label is as follows:
[0035]
[0036] Compound 1N-Boc-4-piperidone (10g, 50mmol) was dissolved in 80ml of anhydrous toluene, added p-toluenesulfonic acid monohydrate (0.3g, 1.5mmol) and tetrahydropyrrole (4.3g, 60mmol), heated Reflux reaction, the water produced by the reaction was separated with an oil-water separator, cooled to room temperature after 3h, added p-toluenesulfonic acid monohydrate (0.3g, 1.5mmol) and ethyl glyoxylate (50% toluene solution) (11ml , 55mmol), heated to reflux for 2h, cooled to room temperature, concentrated the reaction solution to about 20ml, slowly added 4M hydrochloric acid (54ml) dropwise under vigorous stirring, and continued to stir overnight at room temperature. The organic phase was separate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com