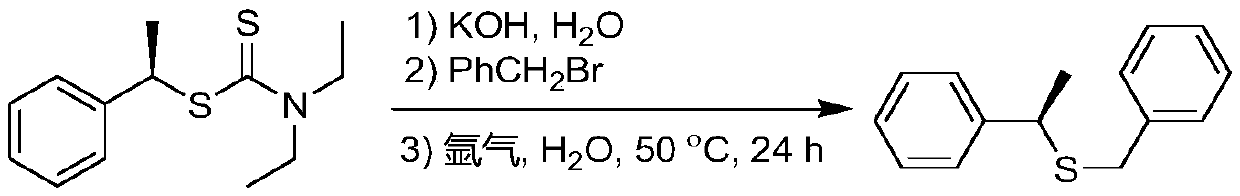Patents
Literature
71 results about "Myocardial imaging" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Myocardial perfusion imaging or scanning (also referred to as MPI or MPS) is a nuclear medicine procedure that illustrates the function of the heart muscle (myocardium).
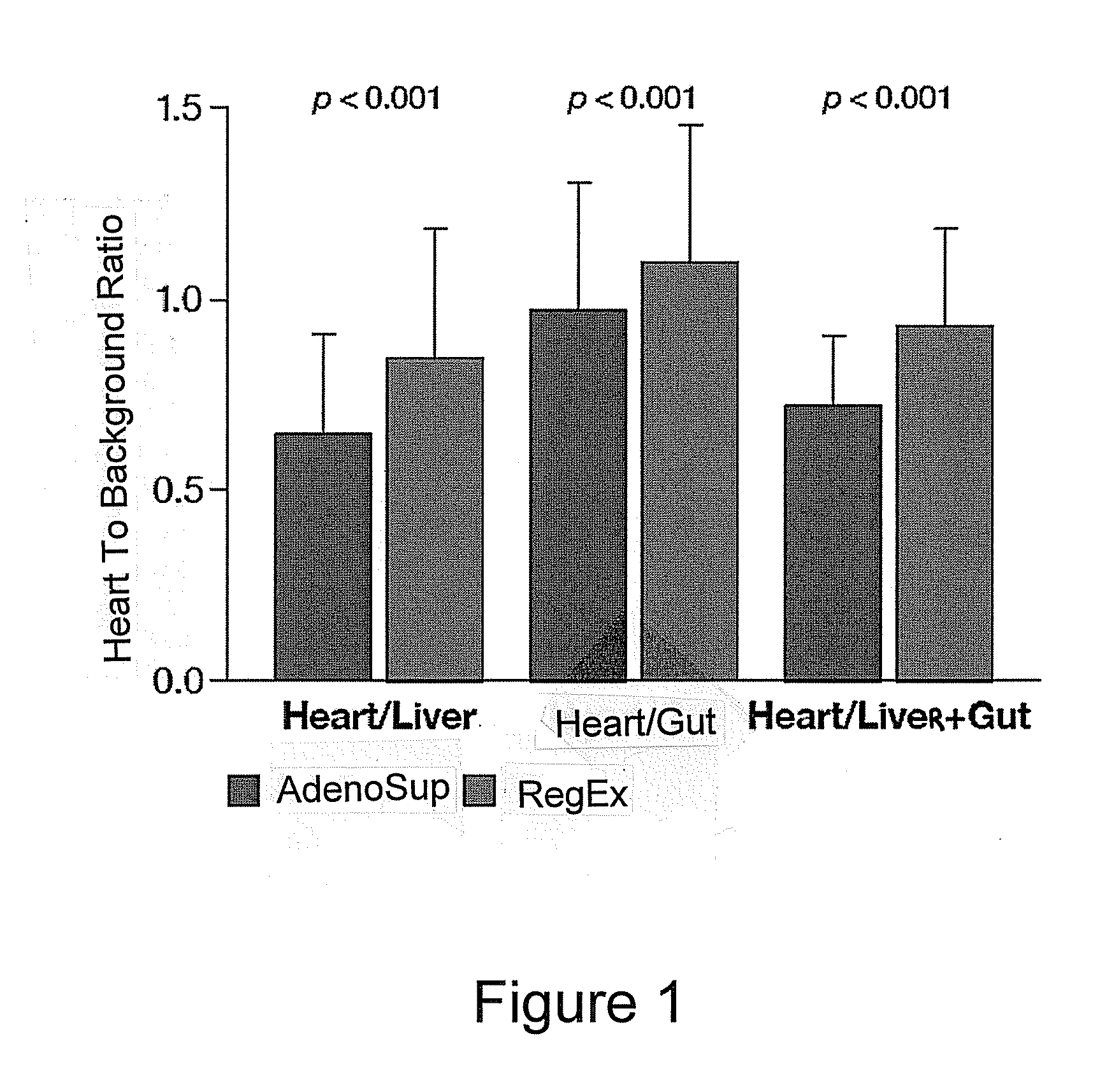
Myocardial perfusion imaging methods and compositions
InactiveUS20050020915A1Facilitate myocardial imagingBiocideCarbohydrate active ingredientsCardiac muscleAgonist
A myocardial imaging method that is accomplished by administering one or more adenosine A2A adenosine receptor agonist to a human undergoing myocardial imaging as well as pharmaceutical compositions comprising at least one A2a receptor agonist, at least one liquid carrier, and at least one co-solvent.
Owner:GILEAD SCI INC +1
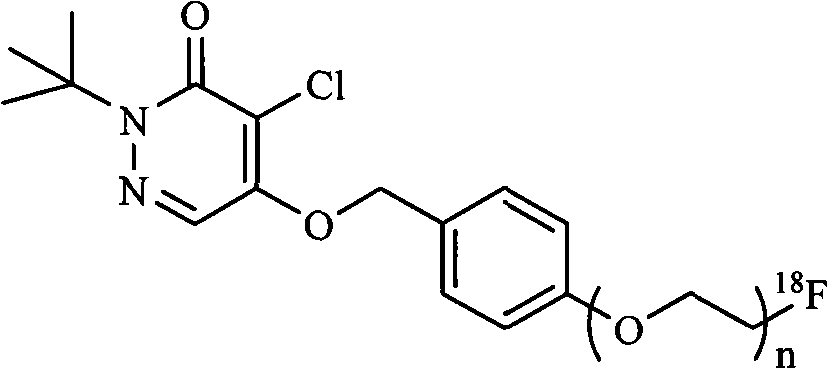
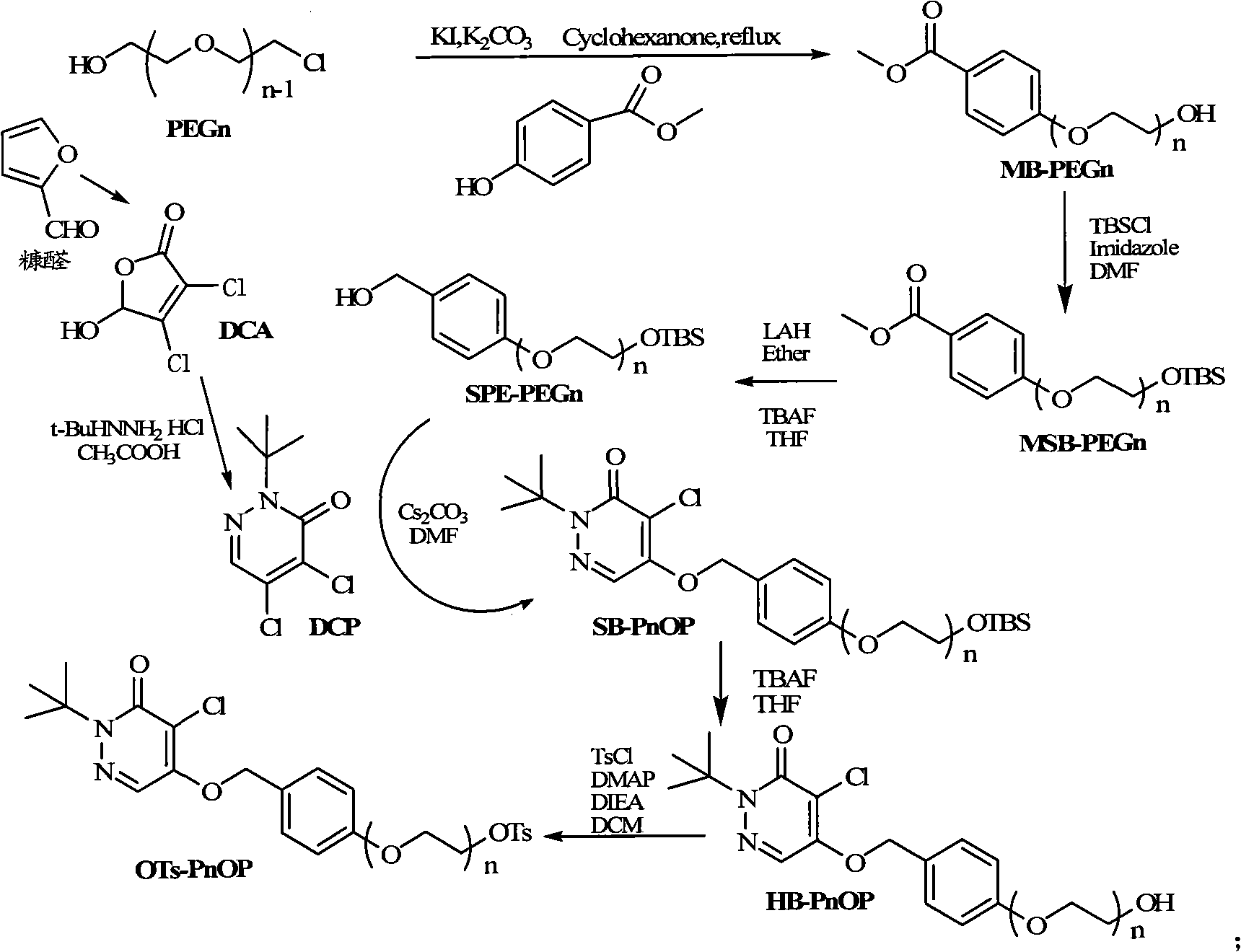
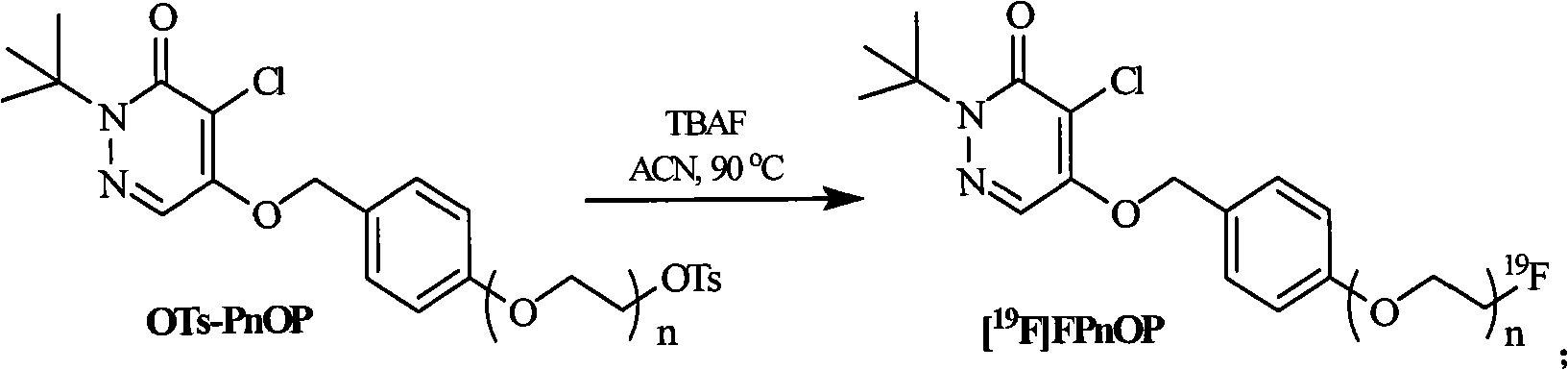
Pyridazinone compound marked by fluorine-18, preparation method and applications
InactiveCN101555232AHigh radiochemical purityGood biological propertiesOrganic chemistryRadioactive preparation carriersBiological propertyRadioactive drug
The invention discloses a pyridazinone compound marked by fluorine-18 with a molecular formula of FFPnOP and a preparation method and applications; in the formula, n is equal to 1, 2 or 3. By technical synthesis to ligand OTs-PnOP, the pyridazinone compound FFPnOP marked by radioactive fluorine-18 and stable reference compound FFPnOP are obtained by synthesis, wherein the stable reference compound is used for confirming the structure of the compound with radioactive marks; the compound has high radiochemical purity, good biological properties, high initial uptake value, simple preparation and low use cost, and is applied in the technical fields of radioactive drug chemistry and clinical nuclear medicine as a novel myocardial perfusion imaging agent marked by fluorine-18.
Owner:BEIJING NORMAL UNIVERSITY +1
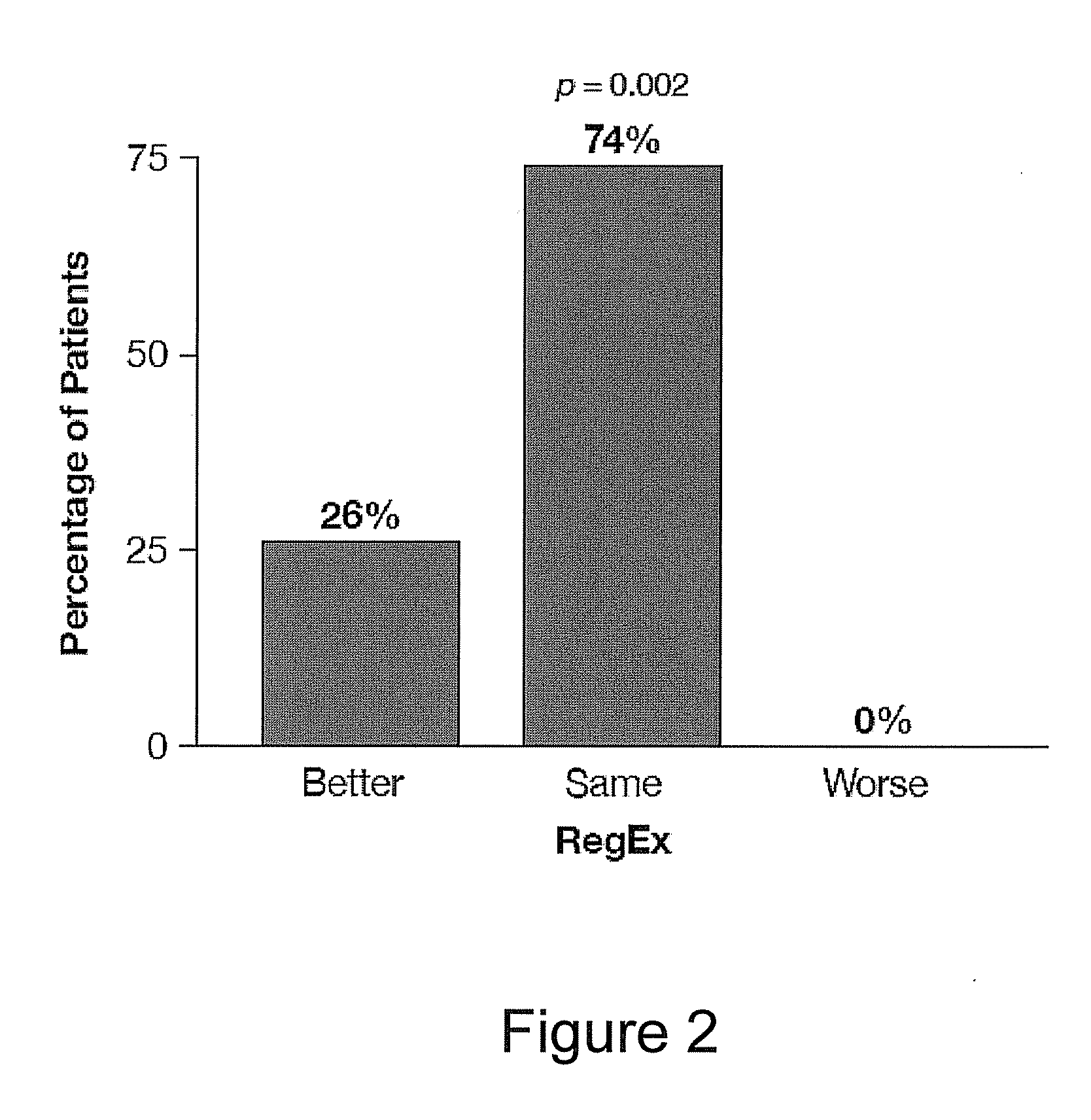
Myocardial Perfusion Imaging
This invention relates to methods for performing myocardial perfusion imaging for diagnosing and characterizing coronary artery disease using an intravenous (IV) bolus injection of regadenoson while the patient is undergoing sub-maximal exercise.
Owner:TPG AXON LEX SUB TRUST +1
Methods and Compositions for Increasing Patient Tolerability During Myocardial Imaging Methods
InactiveUS20090081120A1Facilitate myocardial imagingBiocideRadioactive preparation carriersTolerabilityCardiac muscle
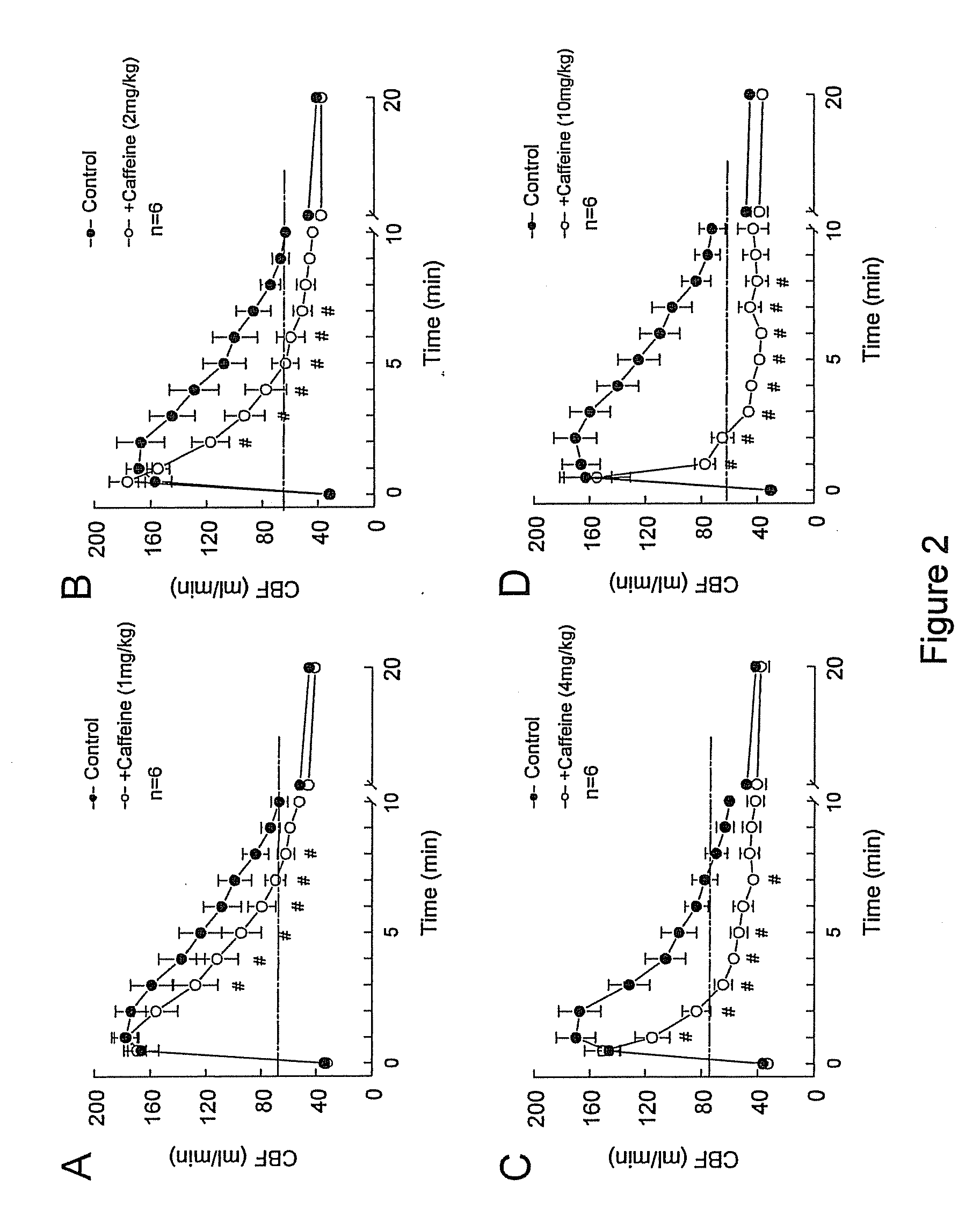
The present application discloses methods and compositions for increasing patient tolerability during myocardial imaging comprising the administration of doses of caffeine and one or more adenosine A2A receptor agonists to a mammal undergoing myocardial imaging.
Owner:GILEAD SCI INC
Method of multidetector computed tomagraphy
InactiveUS20100086483A1Reduce the overall heightCompounds screening/testingBiocideCardiac muscleAdenosine a
This invention relates to methods for multidetector computed tomography myocardial perfusion imaging comprising administering doses of a rate-control agent and one or more adenosine A2A receptor agonists to a mammal.
Owner:GILEAD SCI INC
Methods for Myocardial Imaging in Patients Having a History of Pulmonary Disease
InactiveUS20080170990A1Ultrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsBronchospasmCardiac muscle
The present application discloses methods for myocardial imaging in human patients having a history of pulmonary disease such as asthma, bronchospasm, chronic obstructive pulmonary disease, pulmonary fibrosis, pulmonary inflammation, or pulmonary hypertension, comprising administrating doses of one or more A2A adenosine receptor agonists to a mammal undergoing myocardial imaging and detecting and / or diagnosing myocardial dysfunction.
Owner:TPG AXON LEX SUB TRUST +1
Myocardial perfusion imaging method
InactiveUS7683037B2Facilitate myocardial imagingBiocideIn-vivo radioactive preparationsMedicineCardiac muscle
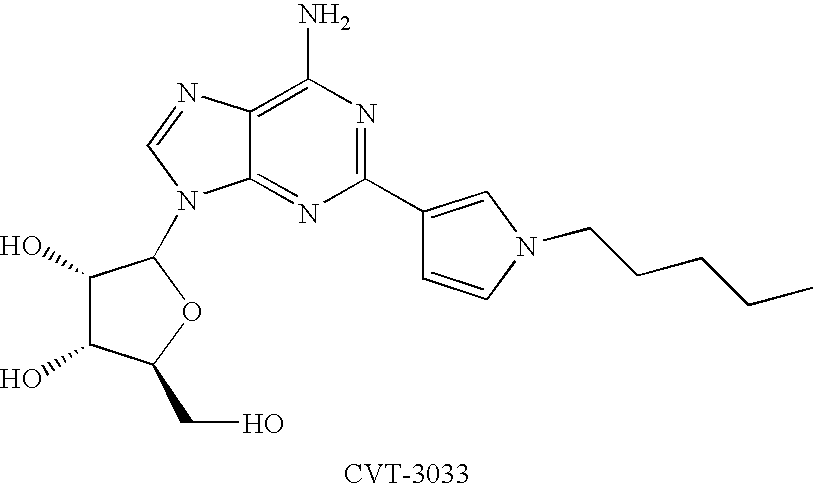
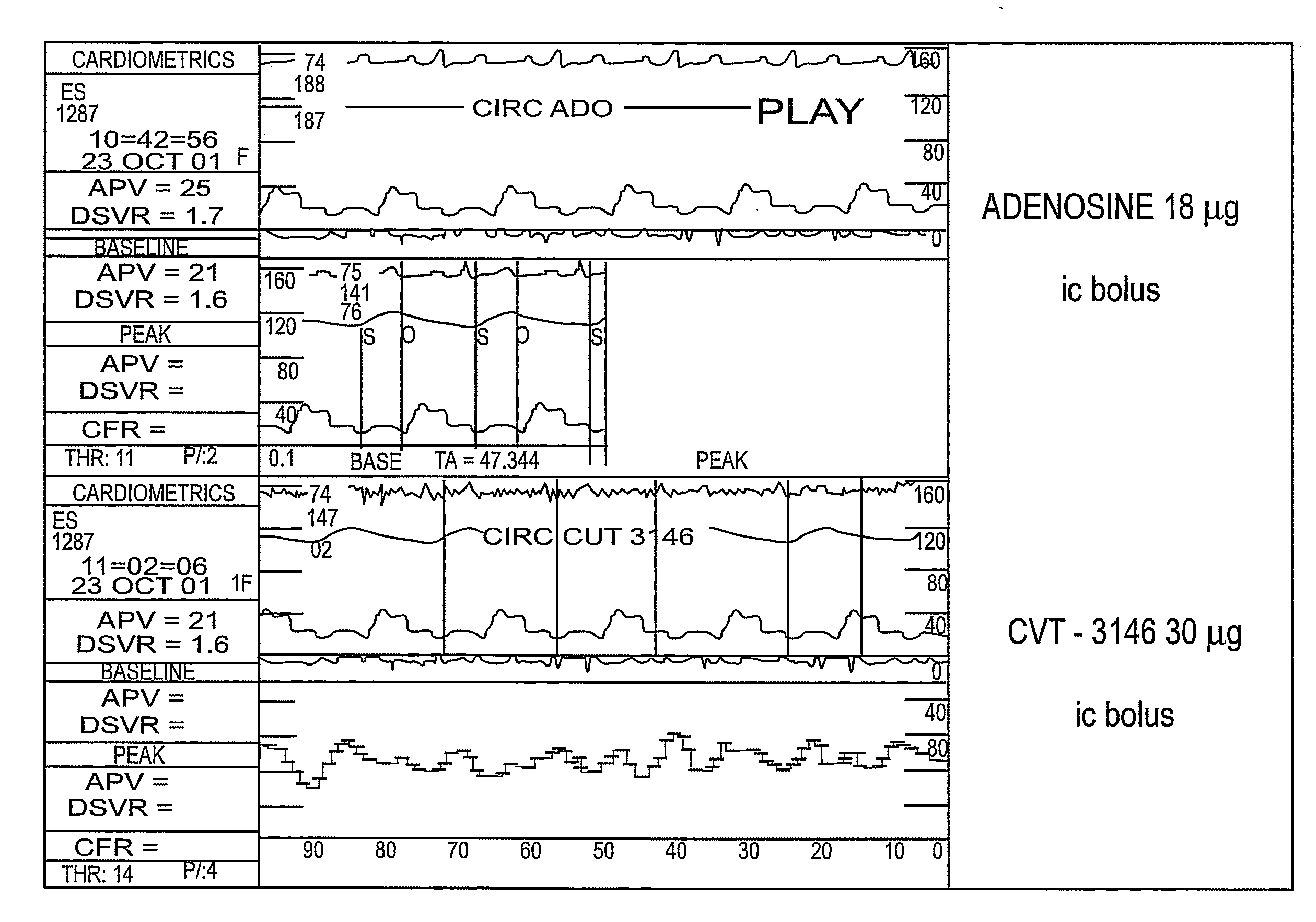
The present invention relates to methods for myocardial imaging by administering at least one 2-adenosine N-pyrazole, 2-adenosine C-pyrazole or a combination thereof A2A adenosine receptor agonist to a human undergoing myocardial imaging. The invention also relates to methods of producing coronary vasodilation without significant peripheral vasodilation by administering at least one 2-adenosine N-pyrazole, 2-adenosine C-pyrazole or a combination thereof adenosine A2A adenosine receptor agonist to a human.
Owner:GILEAD SCI INC +1
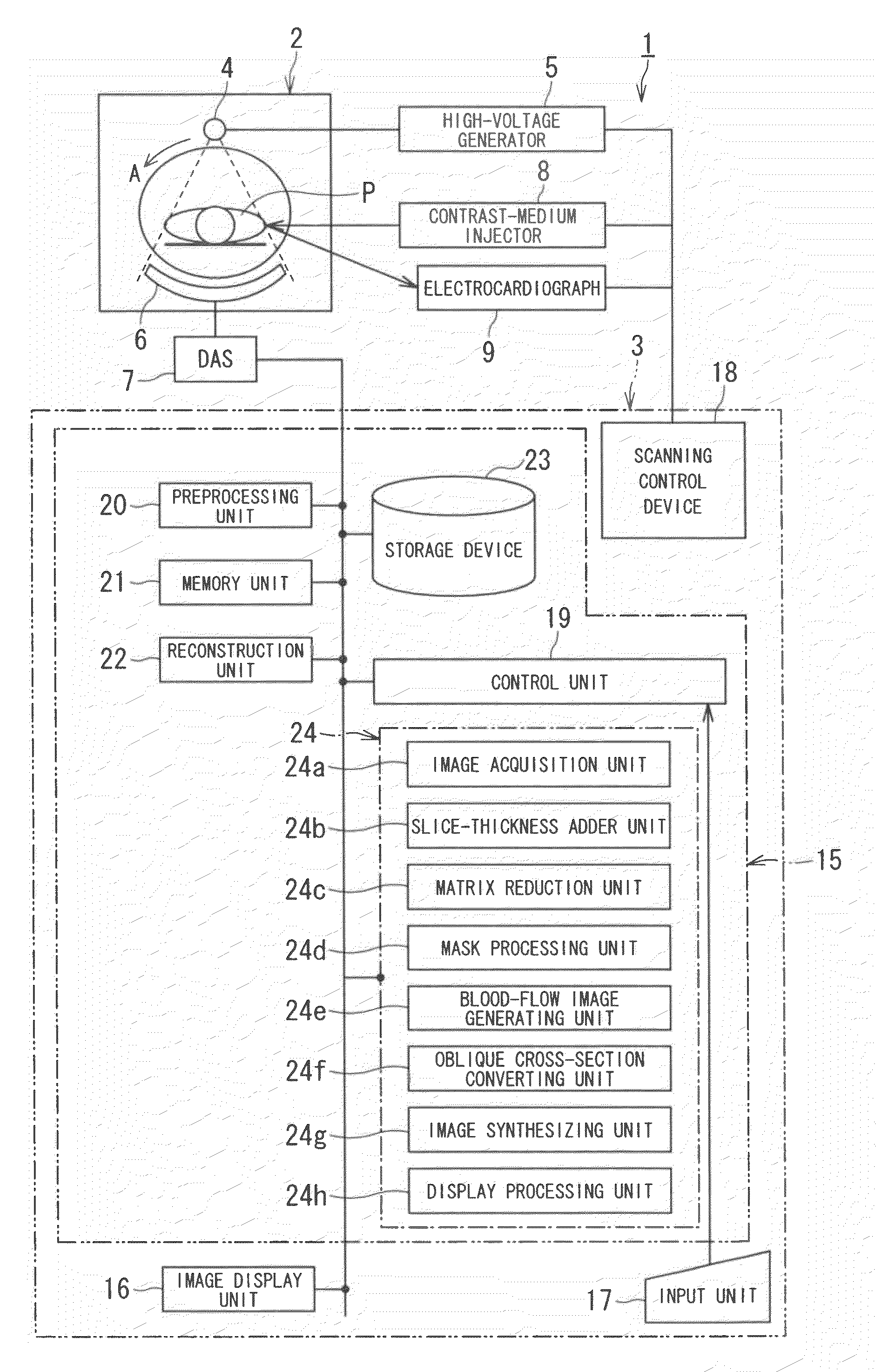
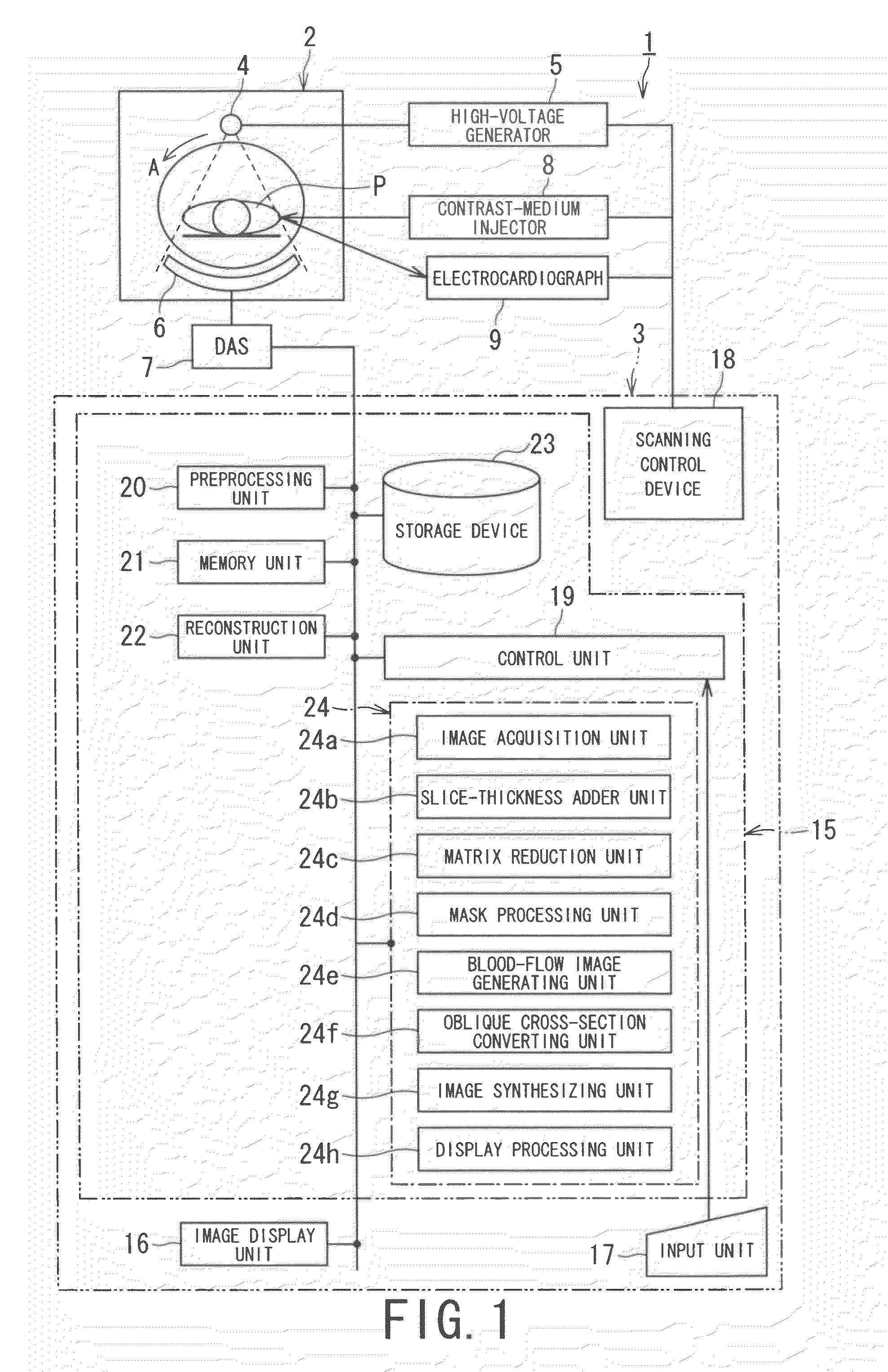
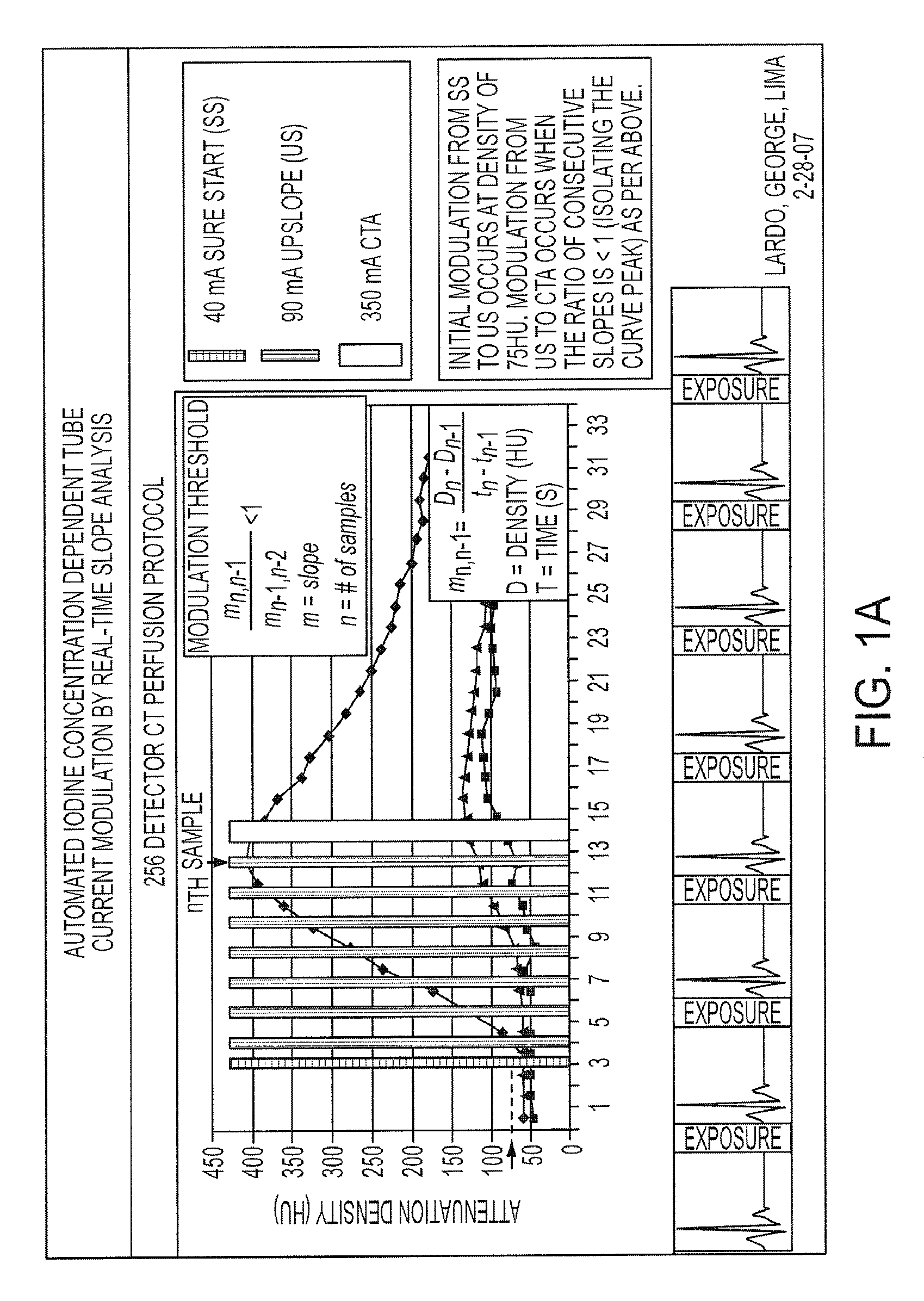
X-ray CT apparatus and myocardial perfusion image generating system
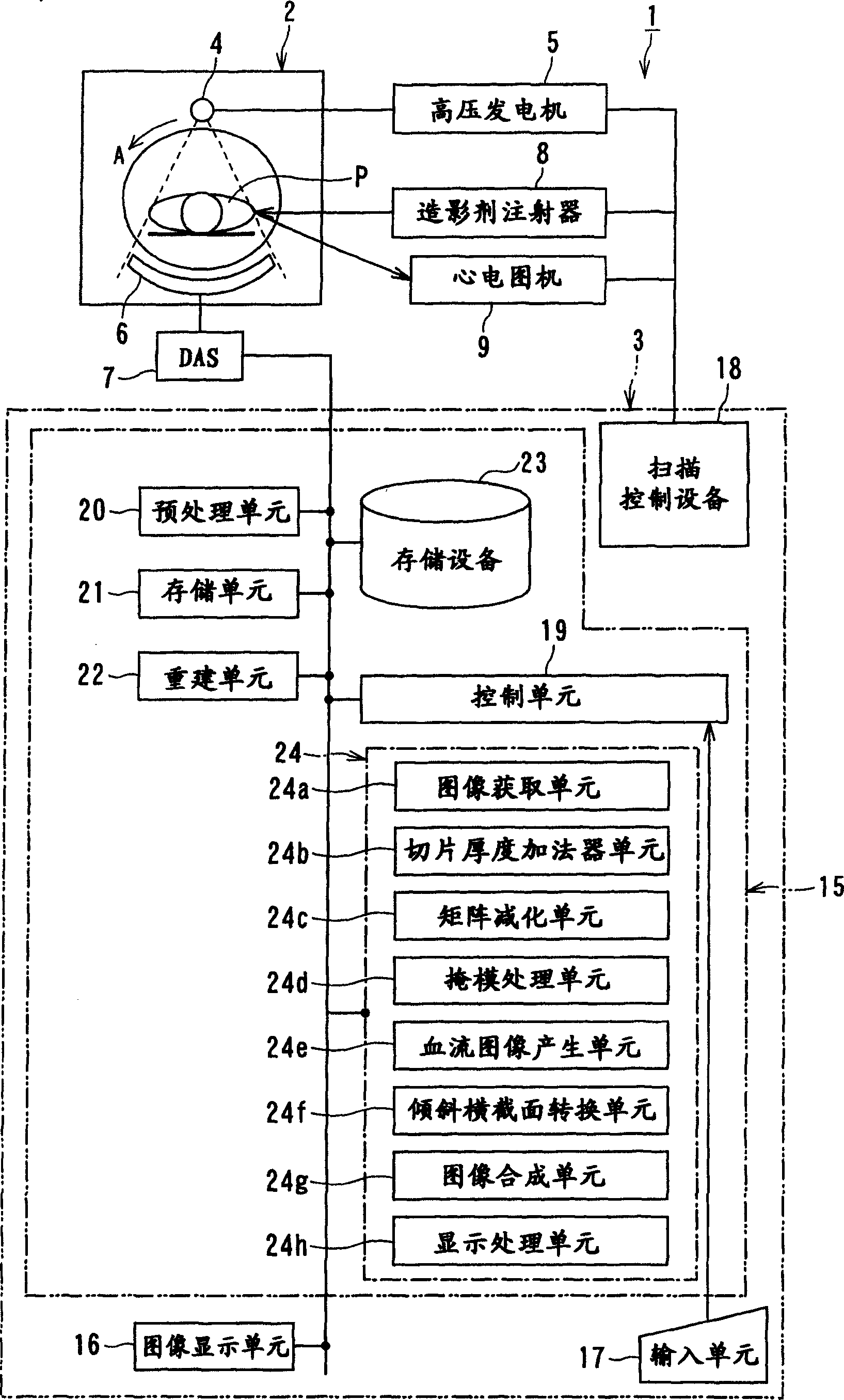
ActiveUS20090129536A1Accurate imagingShort maintenance periodMaterial analysis using wave/particle radiationRadiation/particle handlingHeart muscle extractX-ray
An apparatus includes a transformation table acquiring unit, a blood-flow information acquisition unit and a blood-flow image generating unit. The transformation table acquiring unit obtains a transformation table for transforming unspecified information representing a concentration of a contrast medium in a myocardium into an unspecified blood flow value image based on a CT image acquired in a concentration transition period. The blood-flow information acquisition unit obtains information representing the concentration of the contrast medium based on a CT image acquired during a constant concentration period. The blood-flow image generating unit generates a blood flow value image based on the information representing the concentration of the contrast medium according to the transformation table.
Owner:TOSHIBA MEDICAL SYST CORP +1
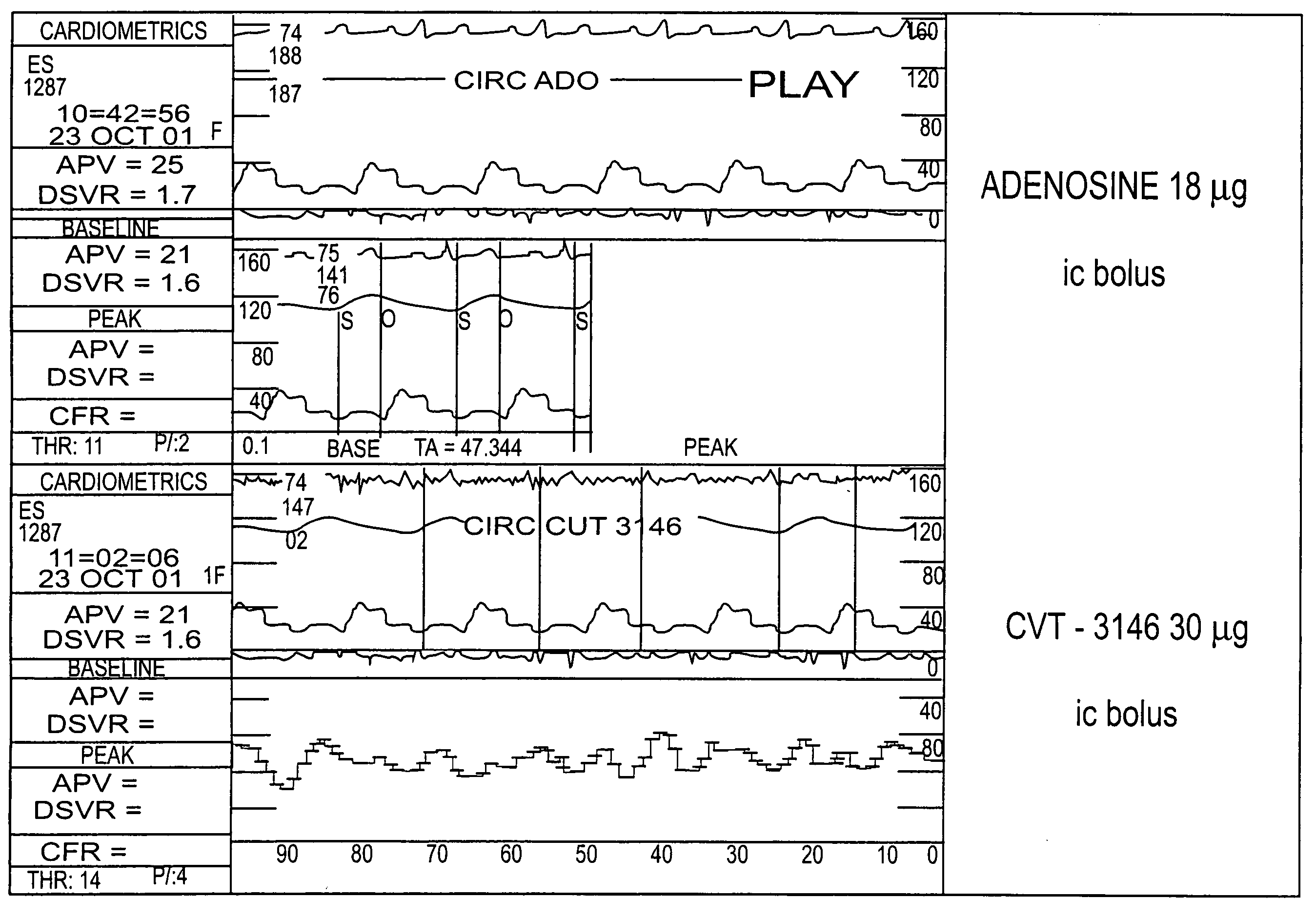
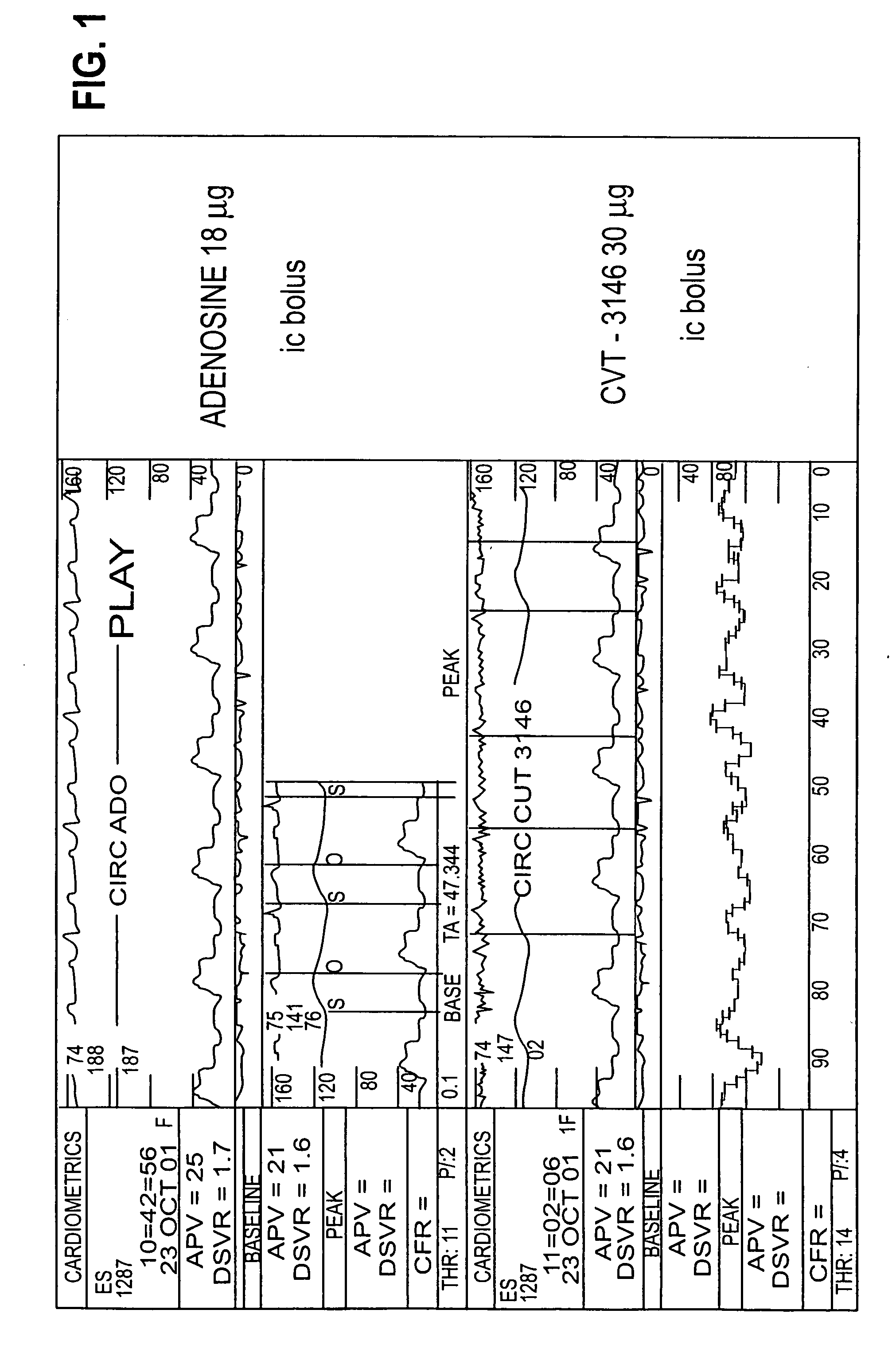
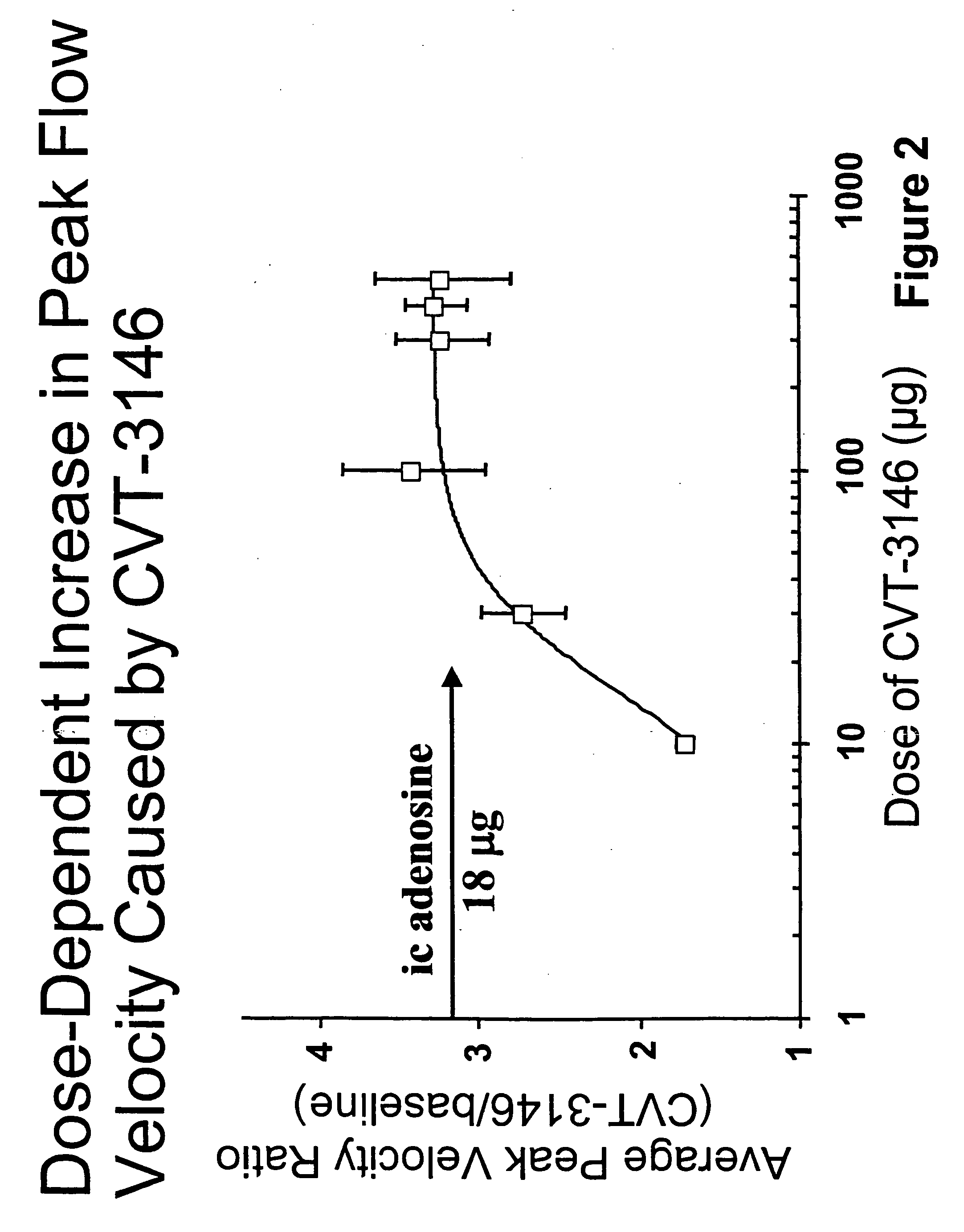
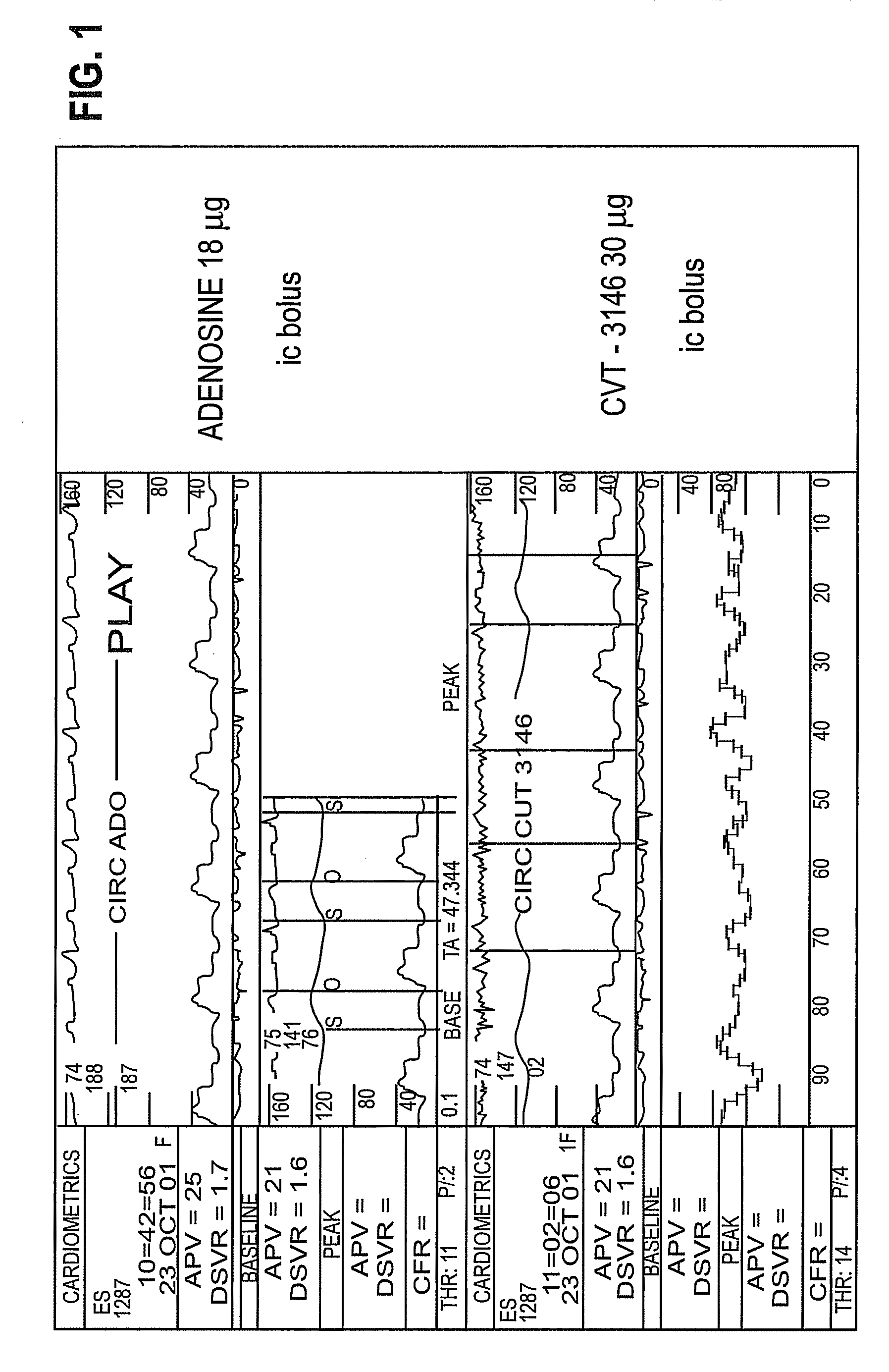
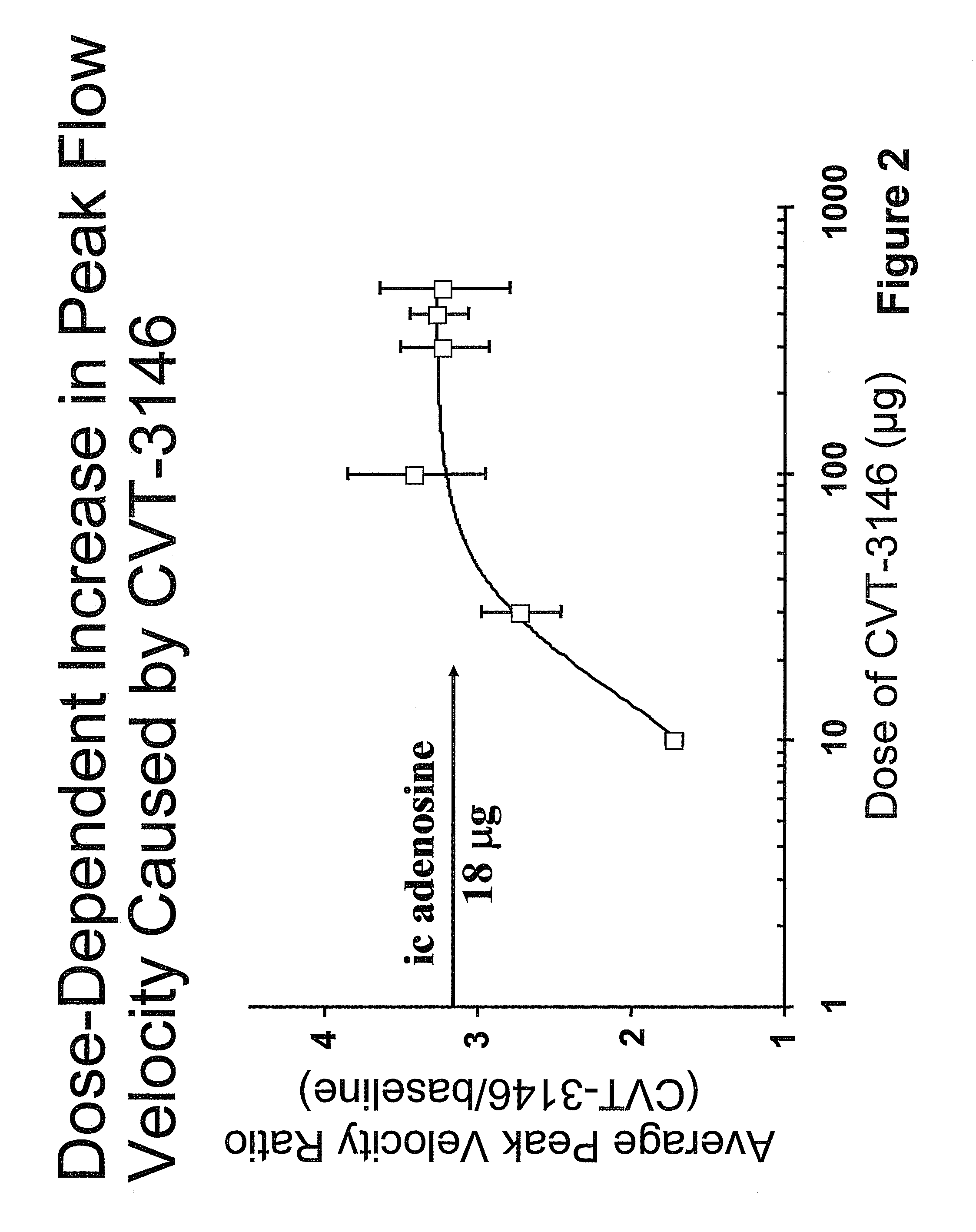
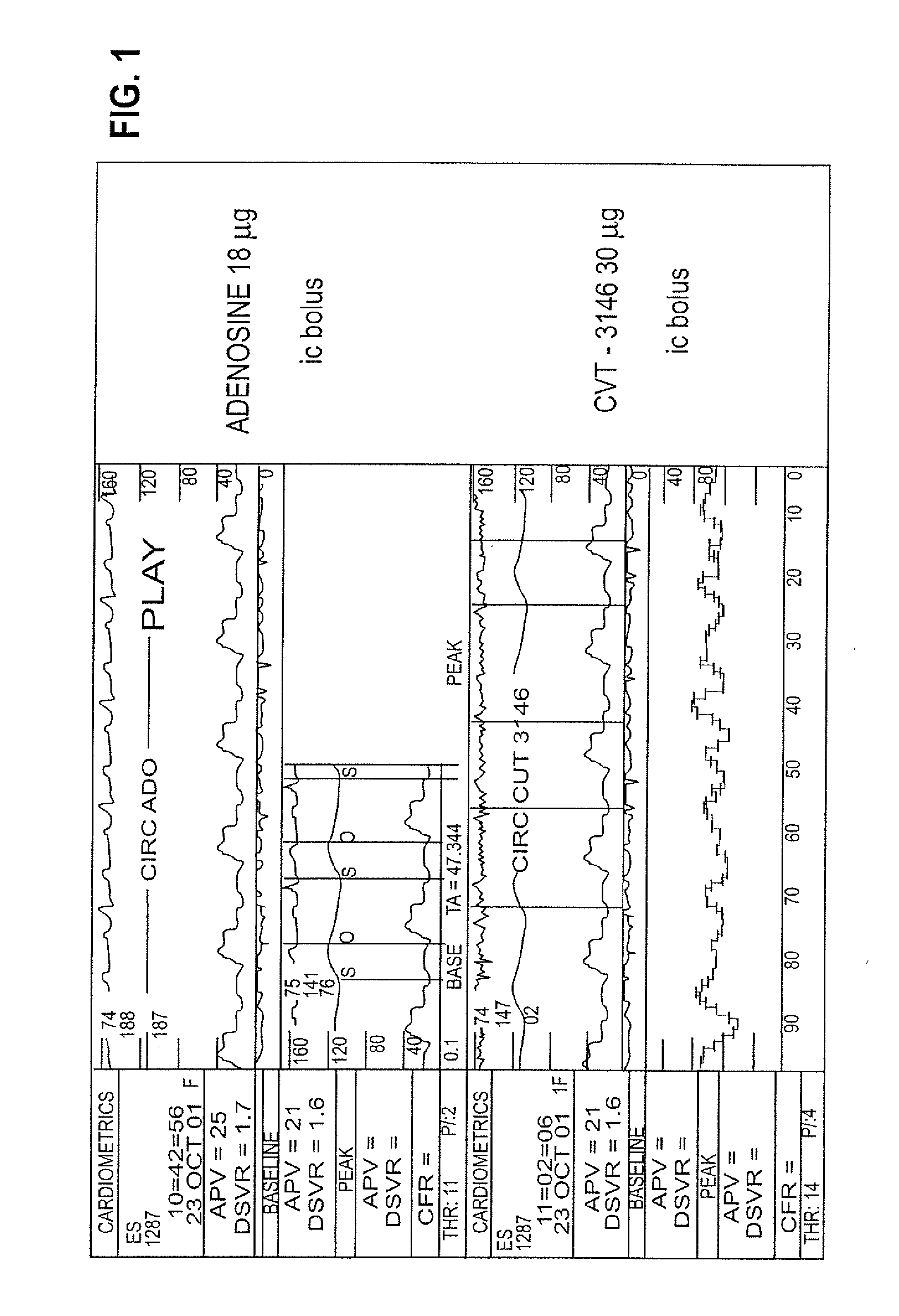
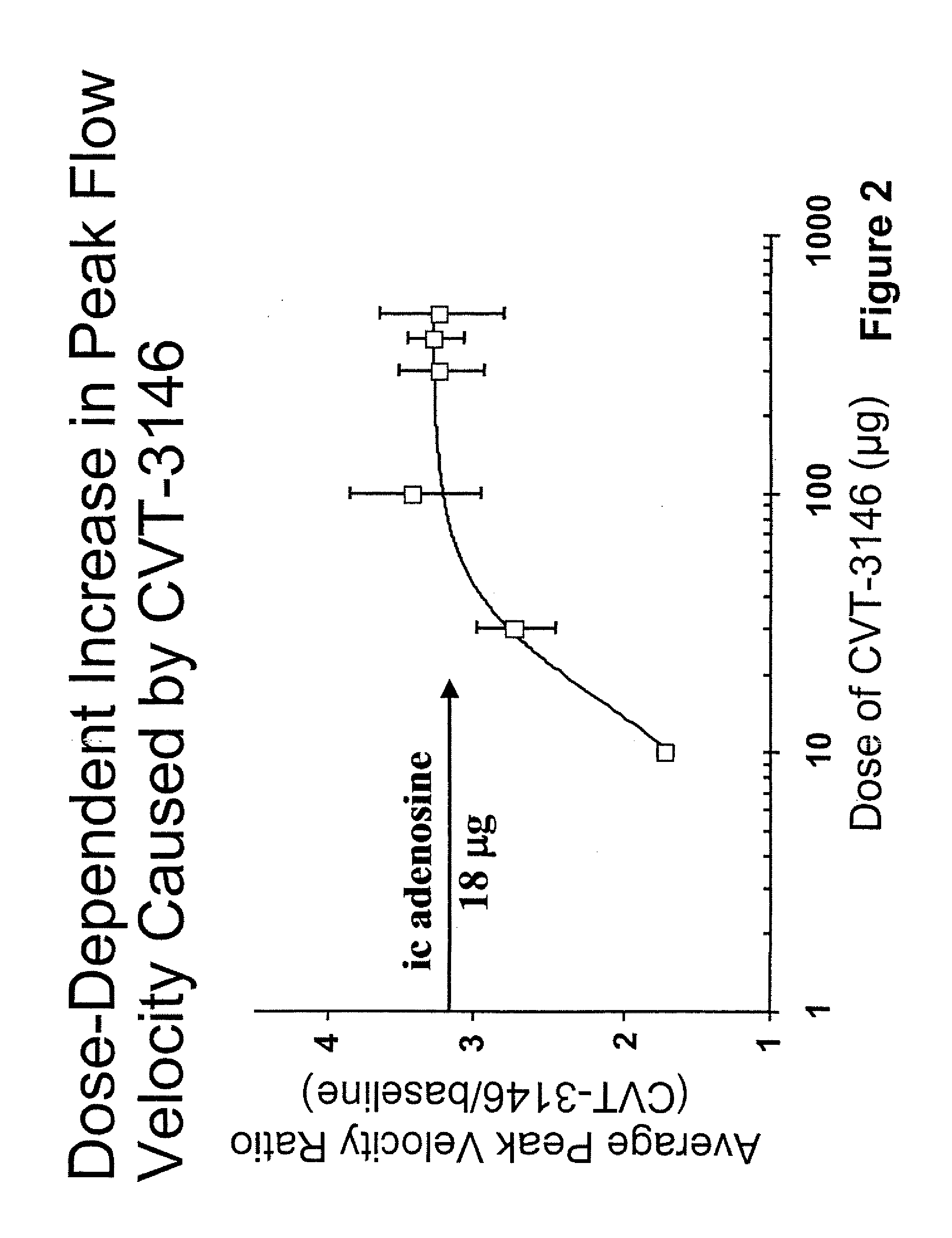
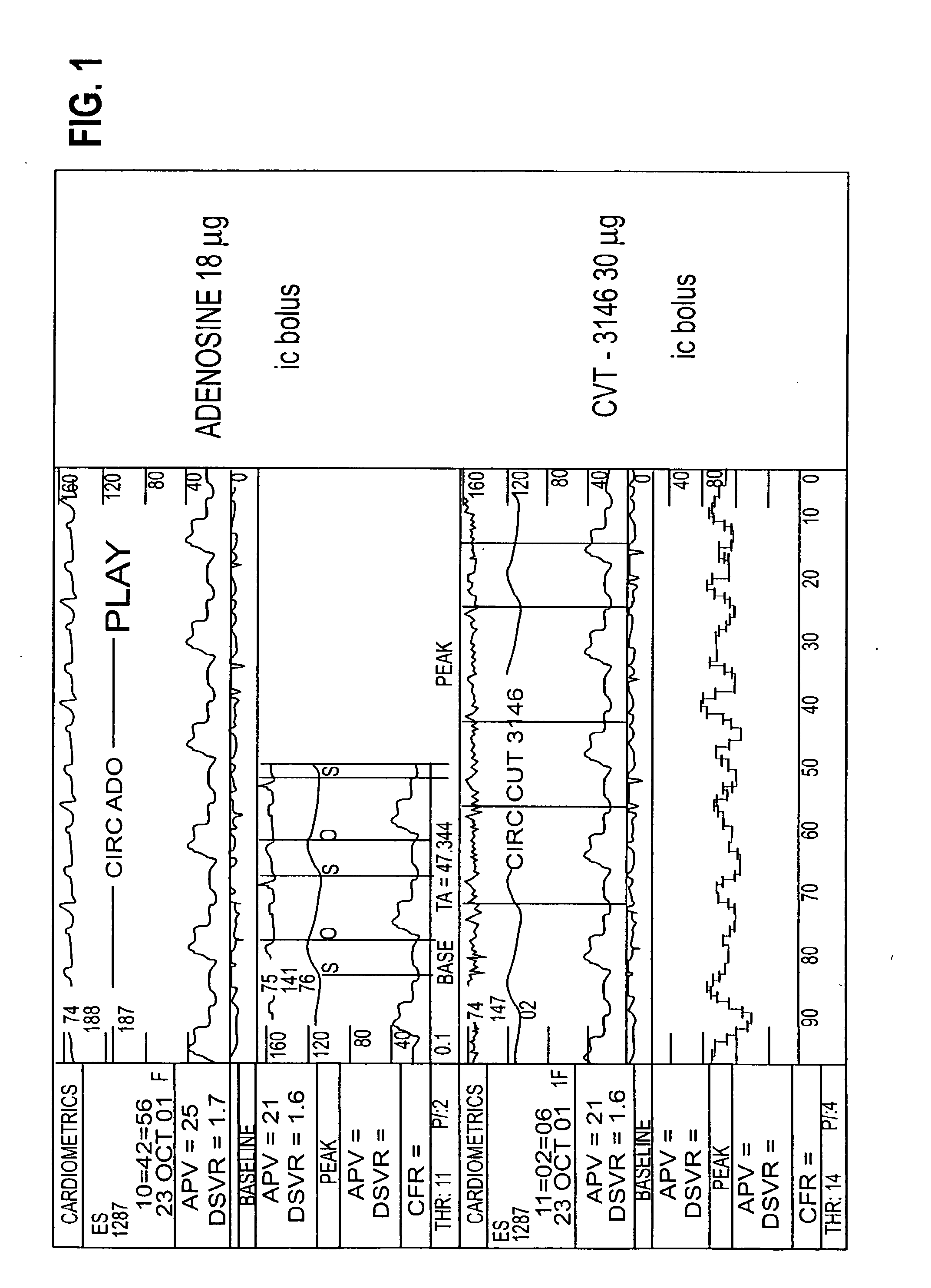
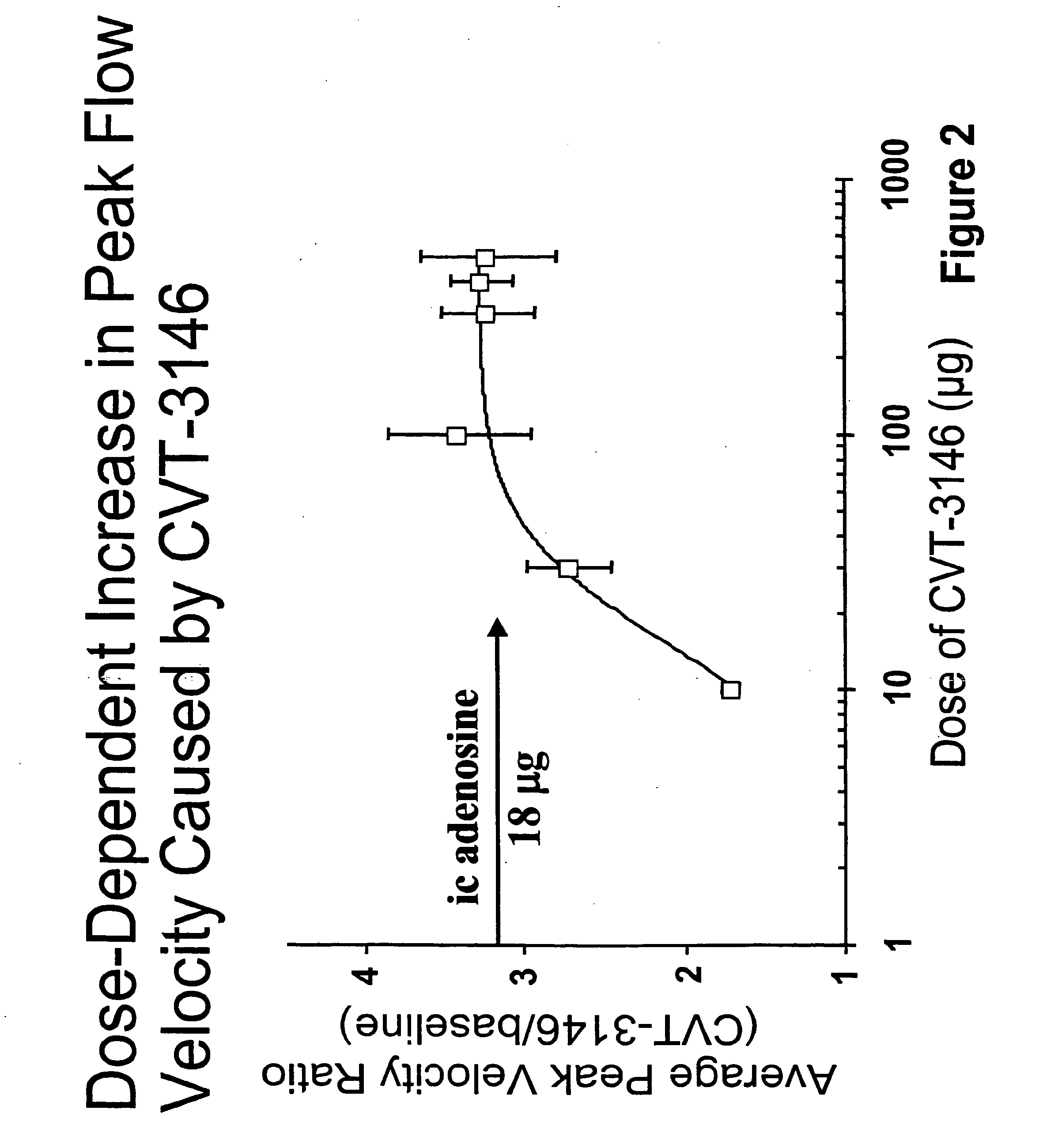
Use of A2A adenosine receptor agonists
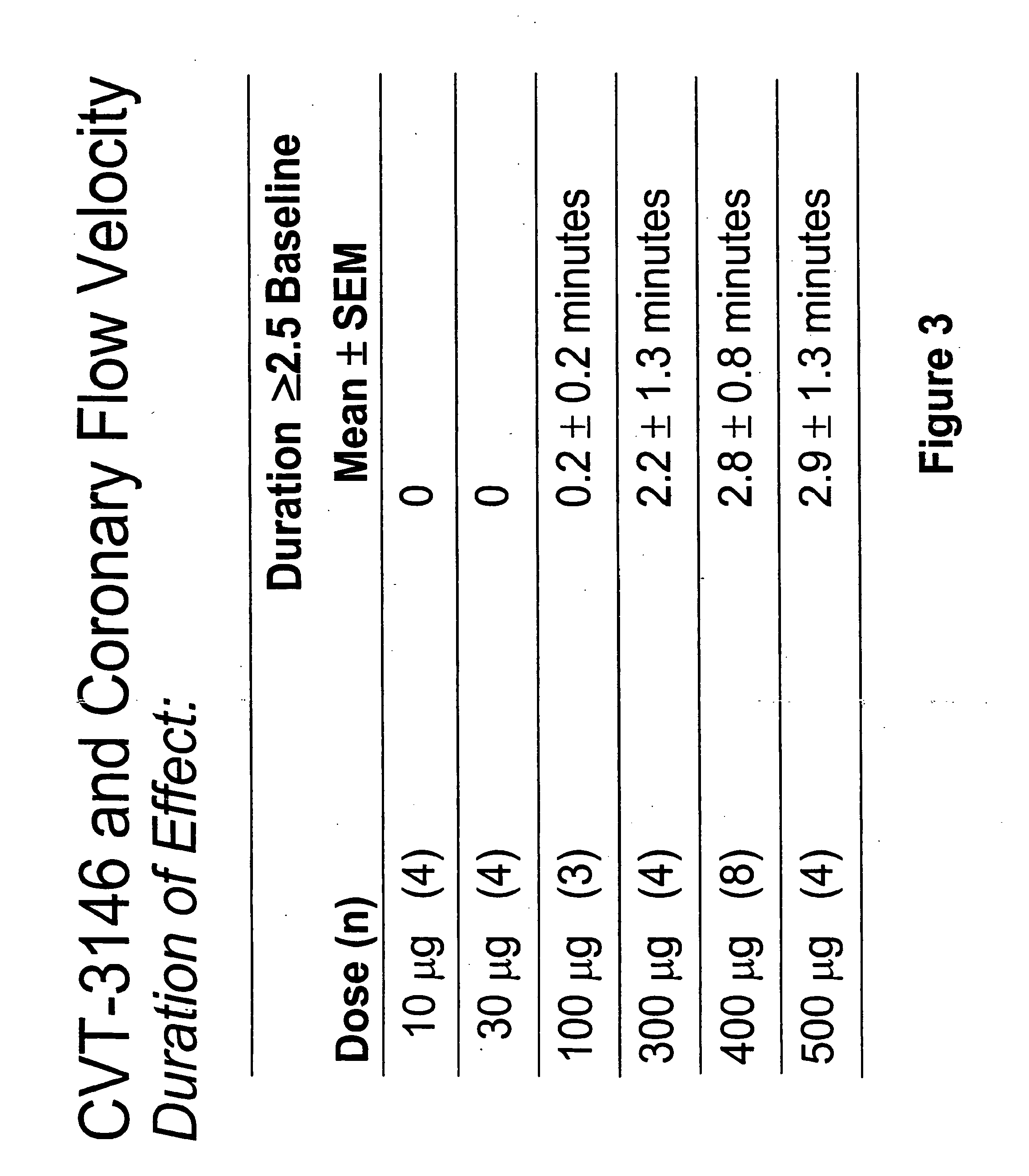
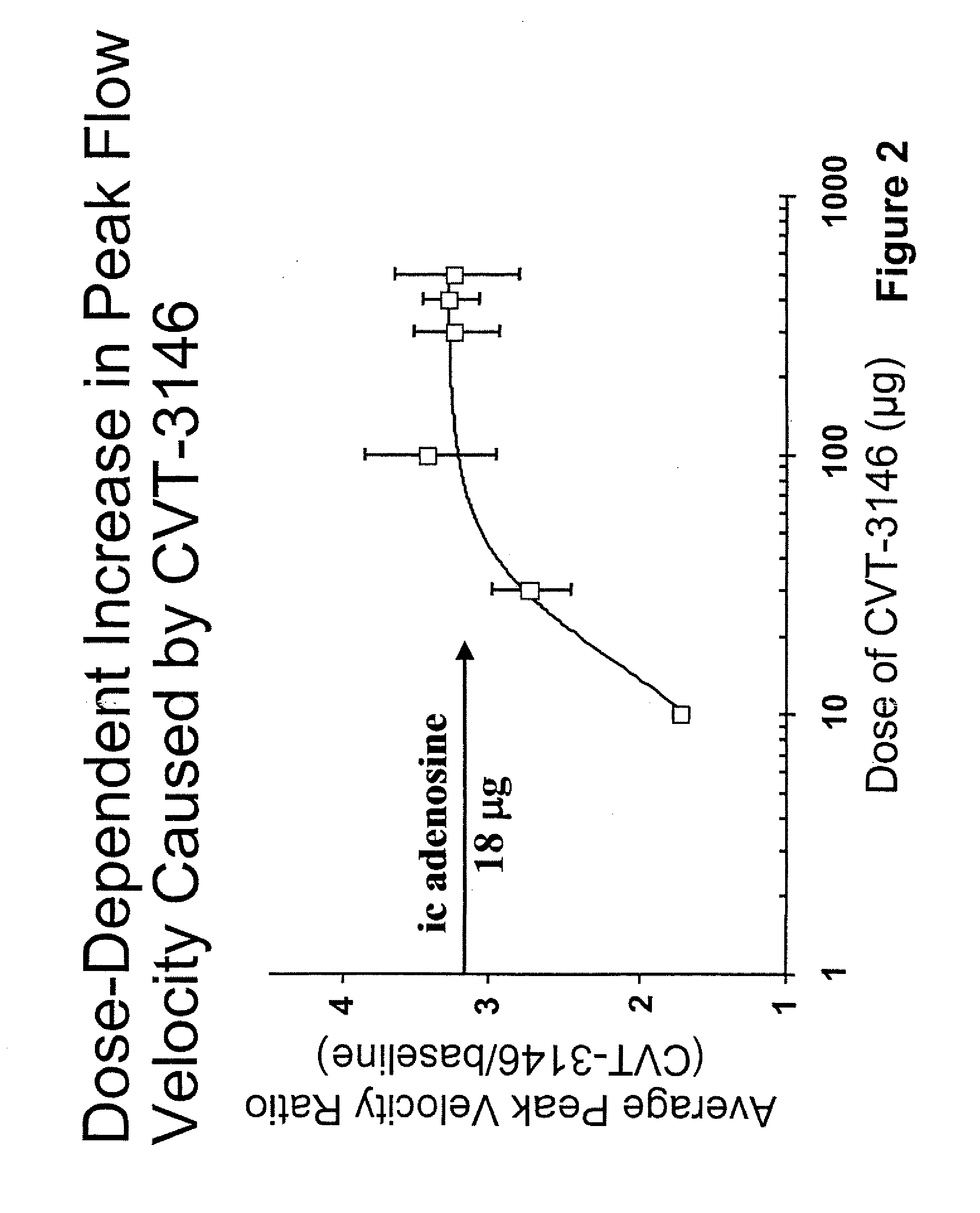
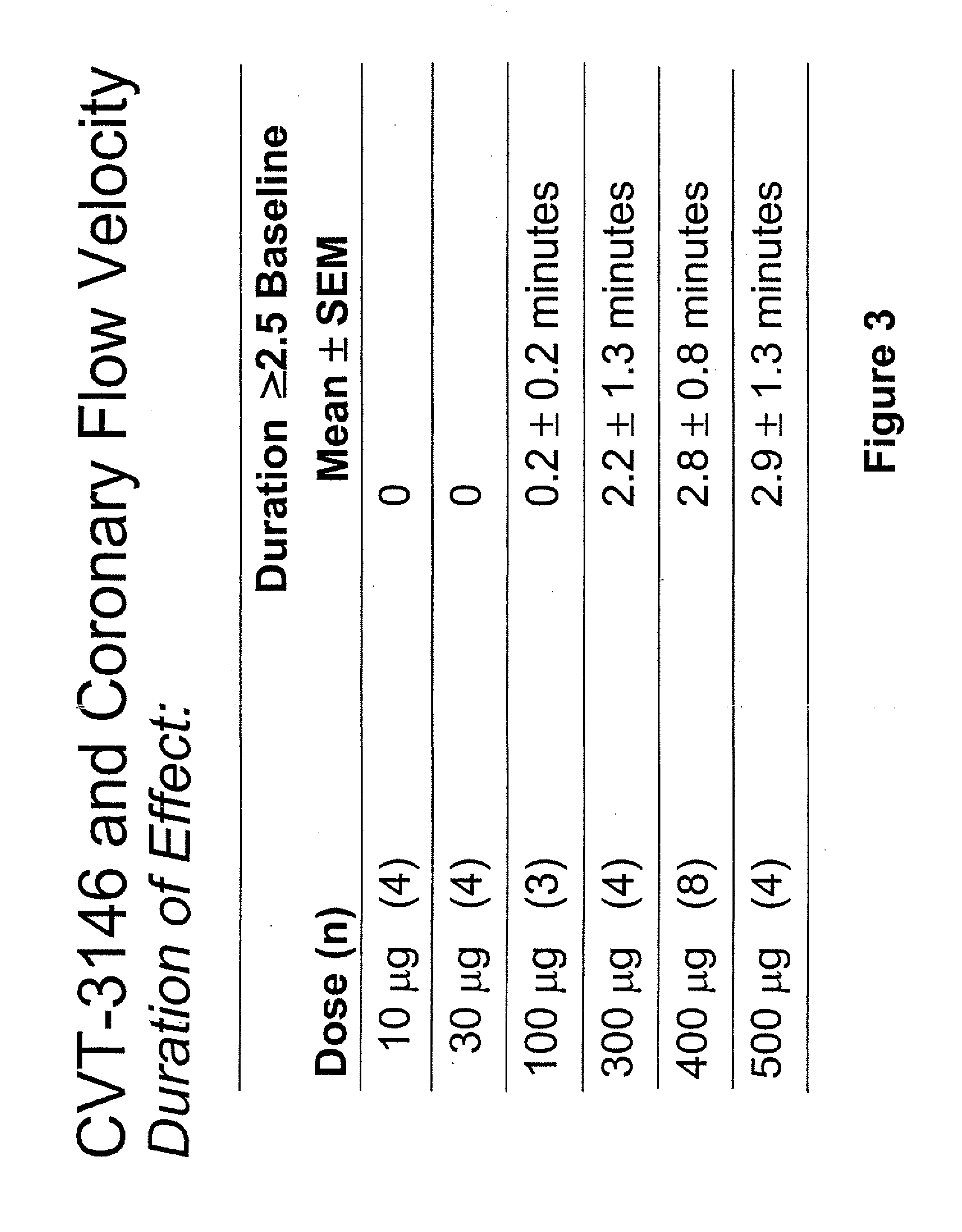
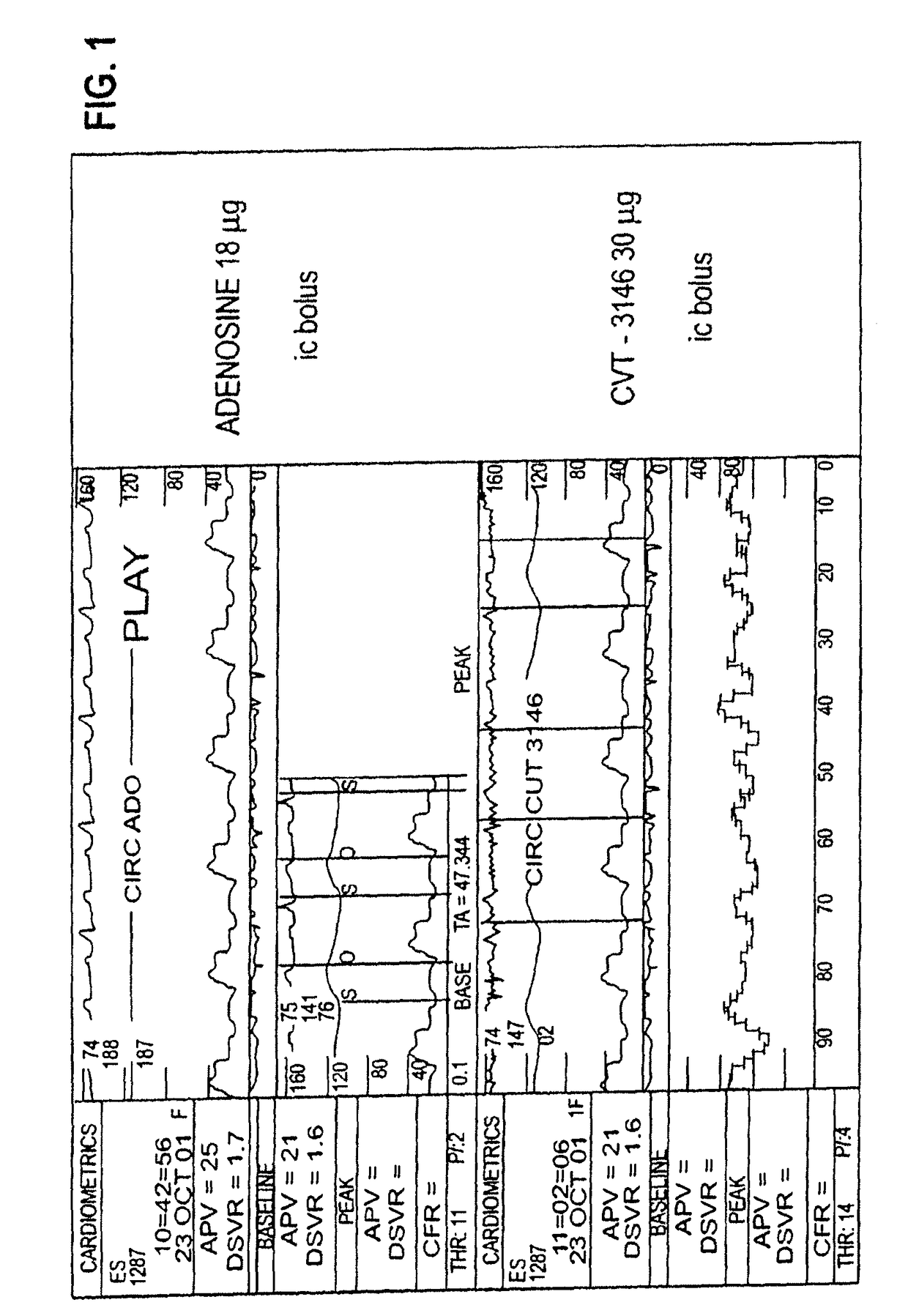
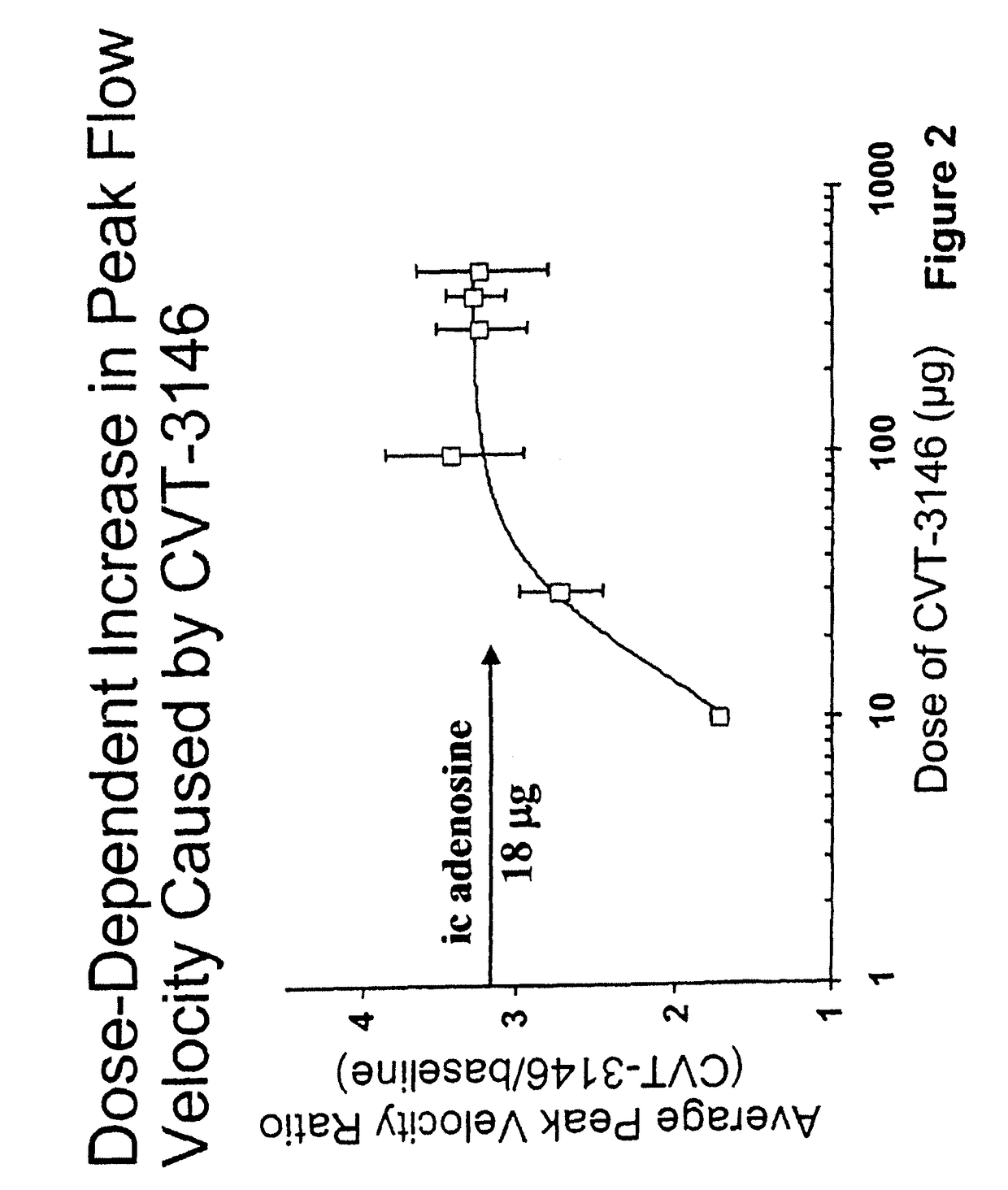
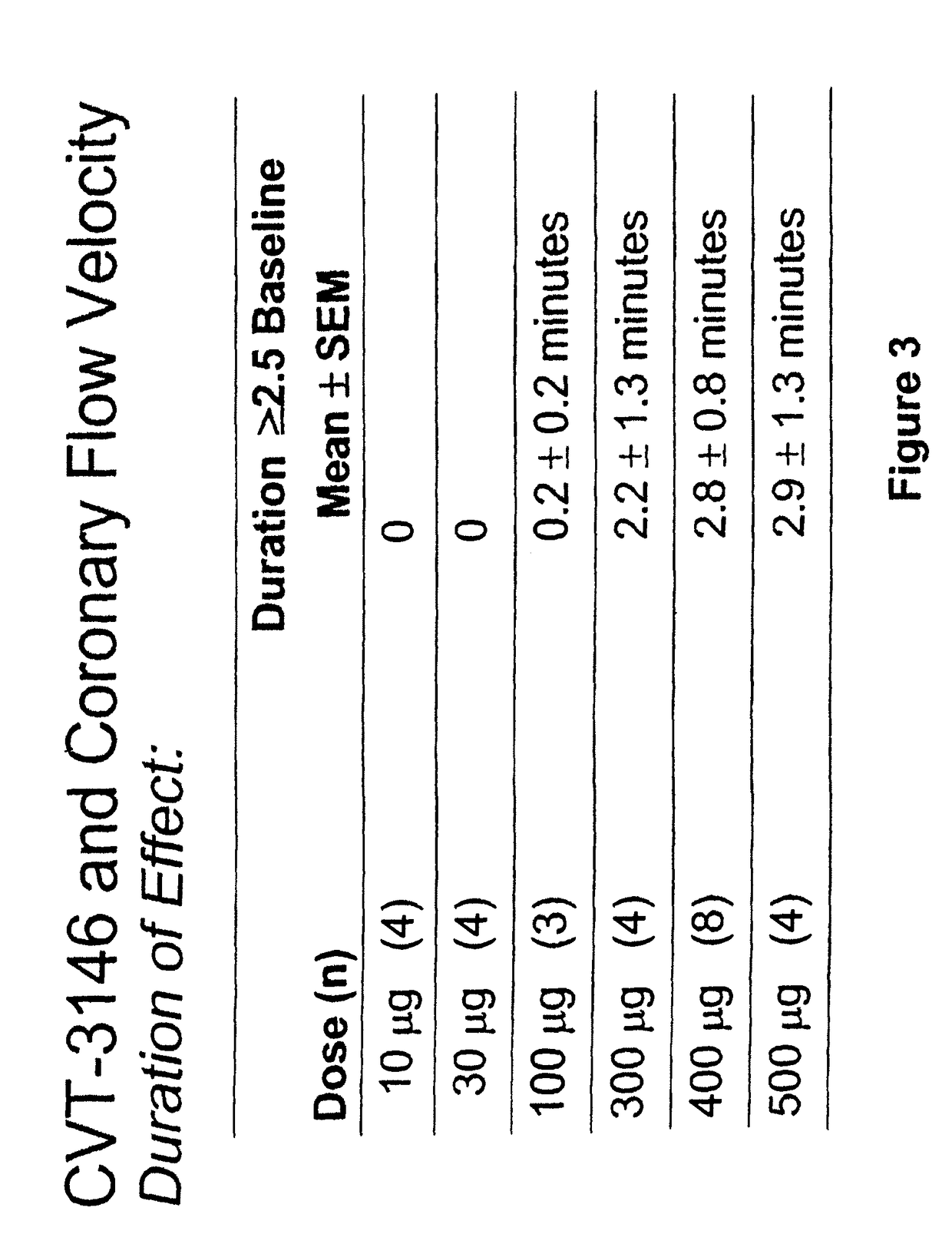
InactiveUS20060084625A1Increase peak flow velocityBiocideEchographic/ultrasound-imaging preparationsRegadenosonCardiac muscle
Myocardial imaging methods that are accomplished by administering doses of a pharmaceutical composition including regadenoson—an adenosine A2A receptor agonist—to a human undergoing myocardial imaging in an amount sufficient to achieve at least a minimal increase in average coronary peak flow velocity.
Owner:TPG AXON LEX SUB TRUST +1
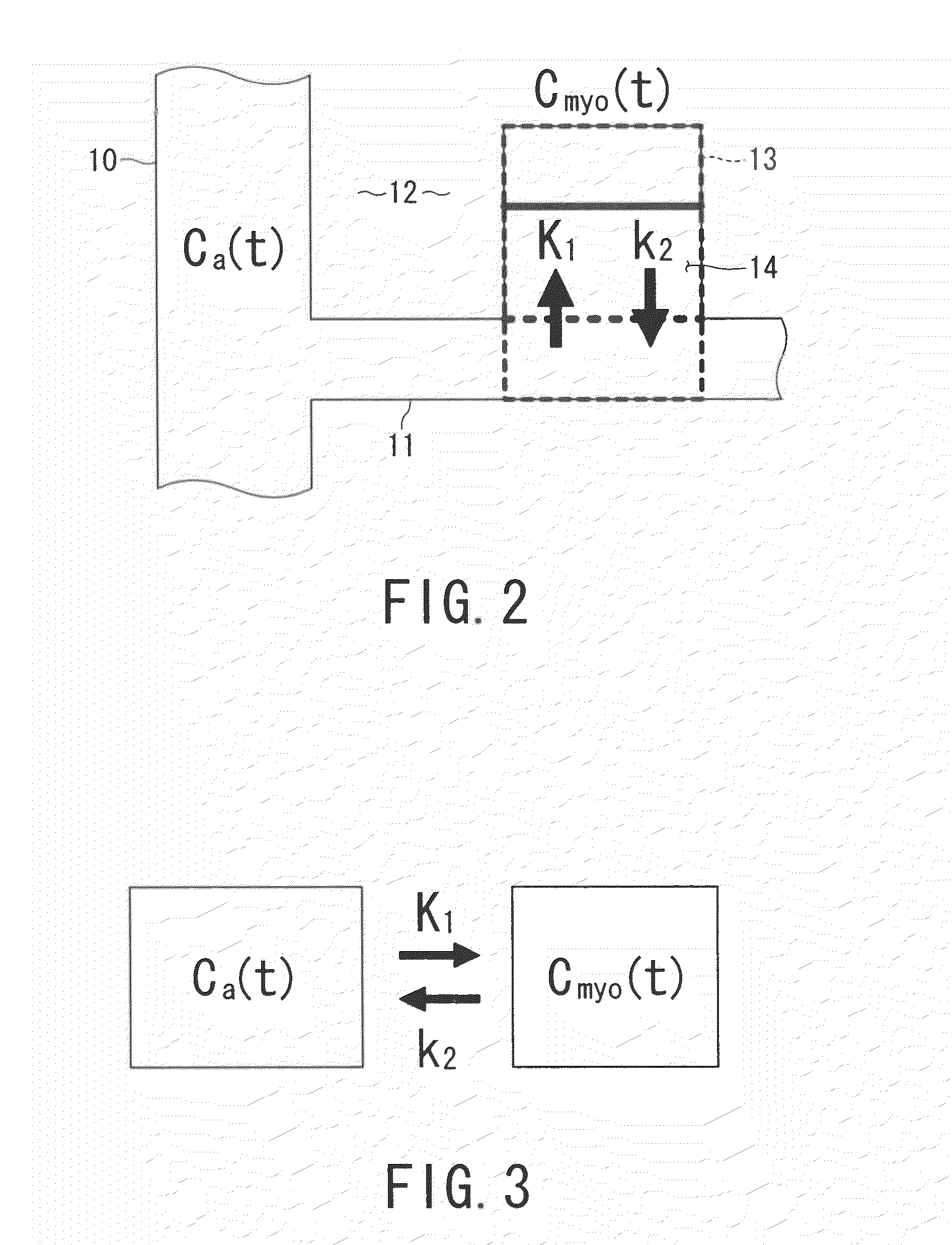
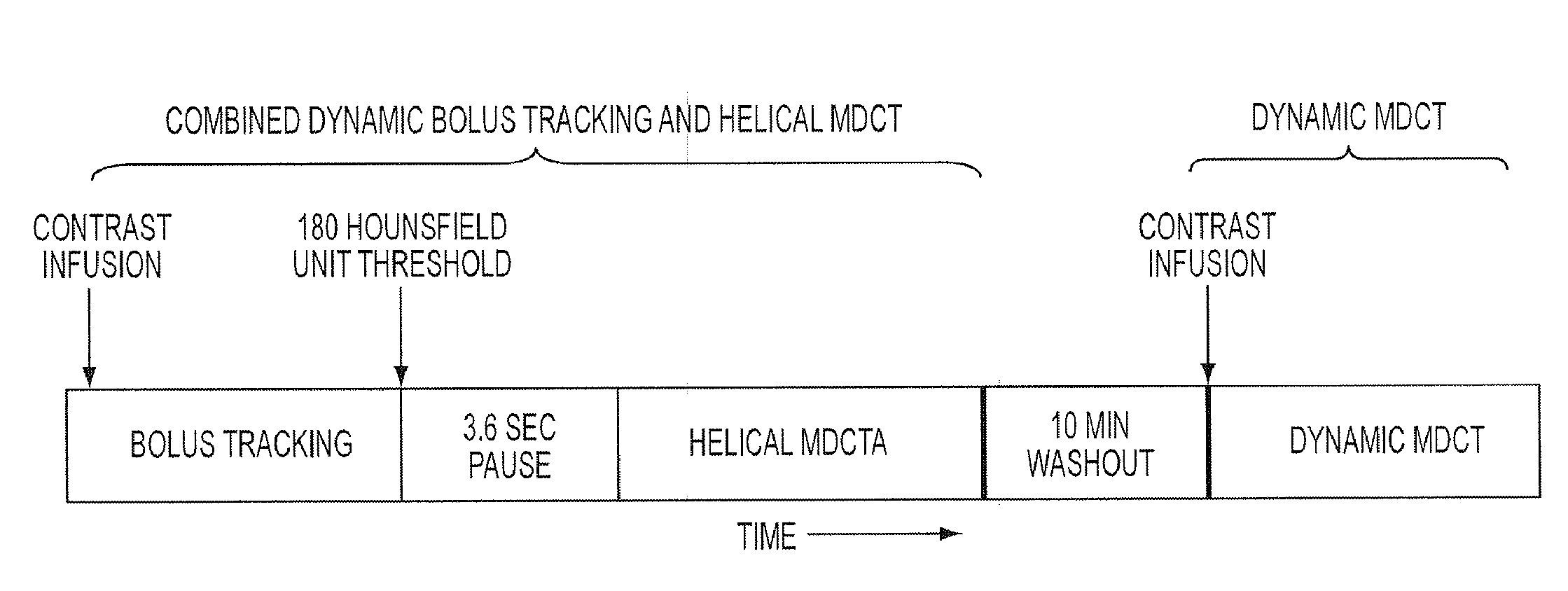
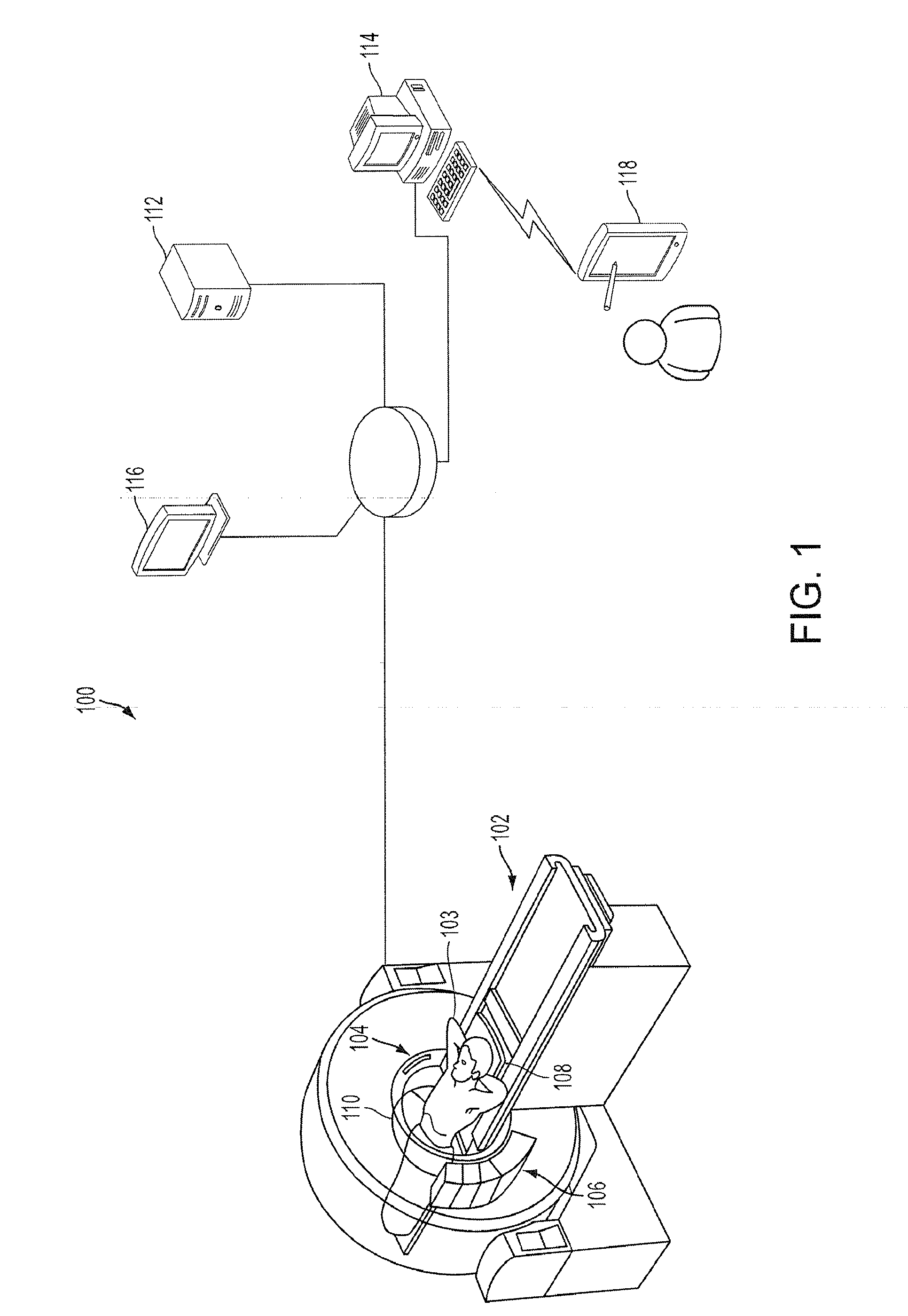
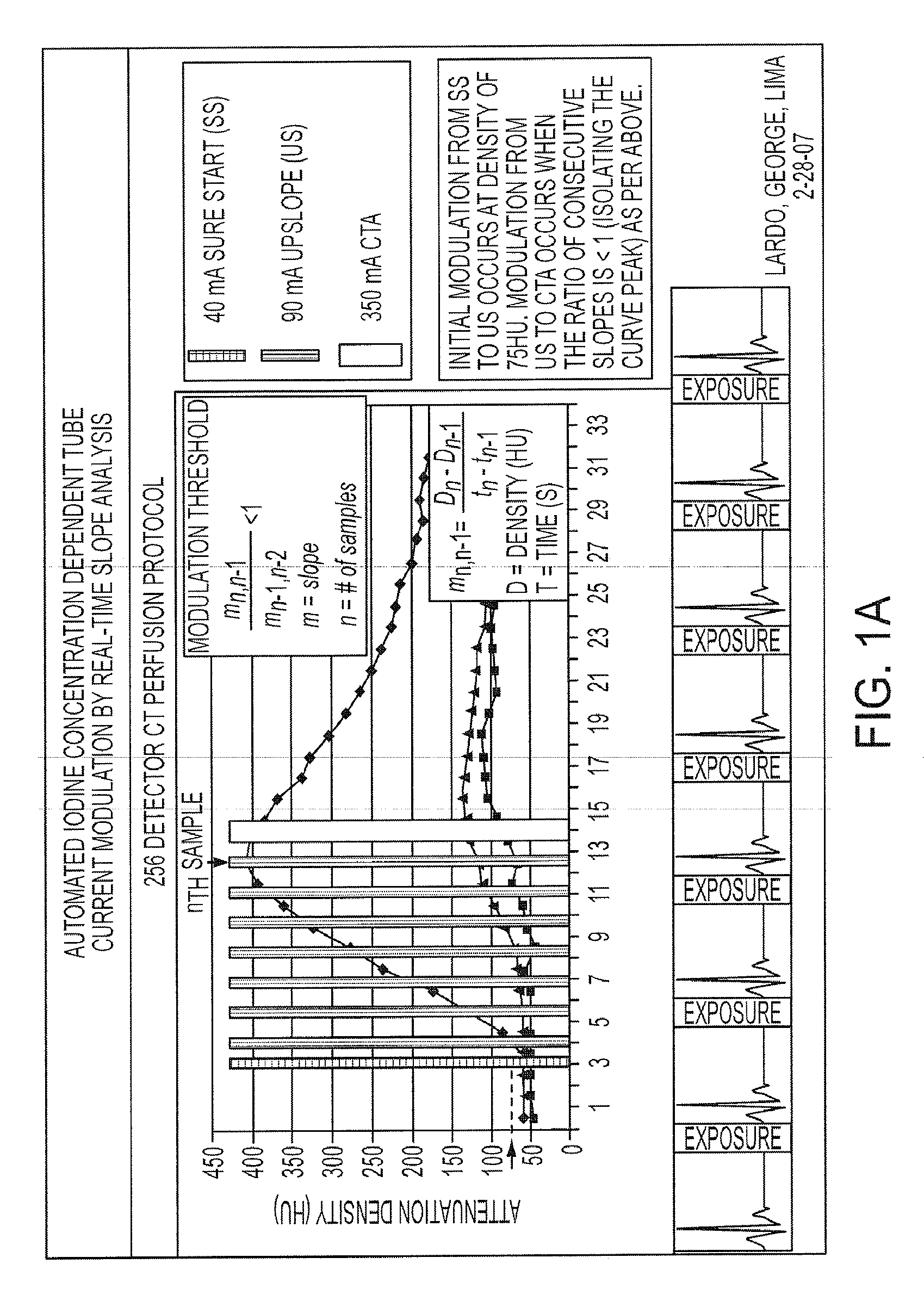
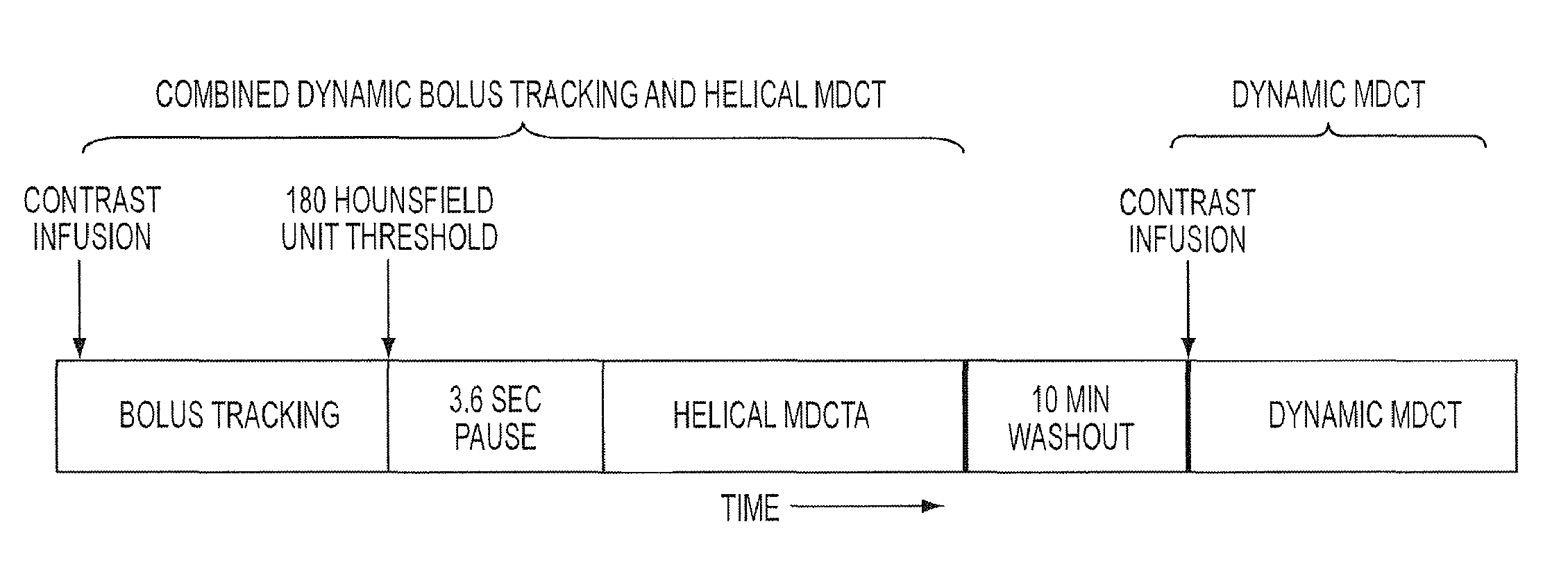
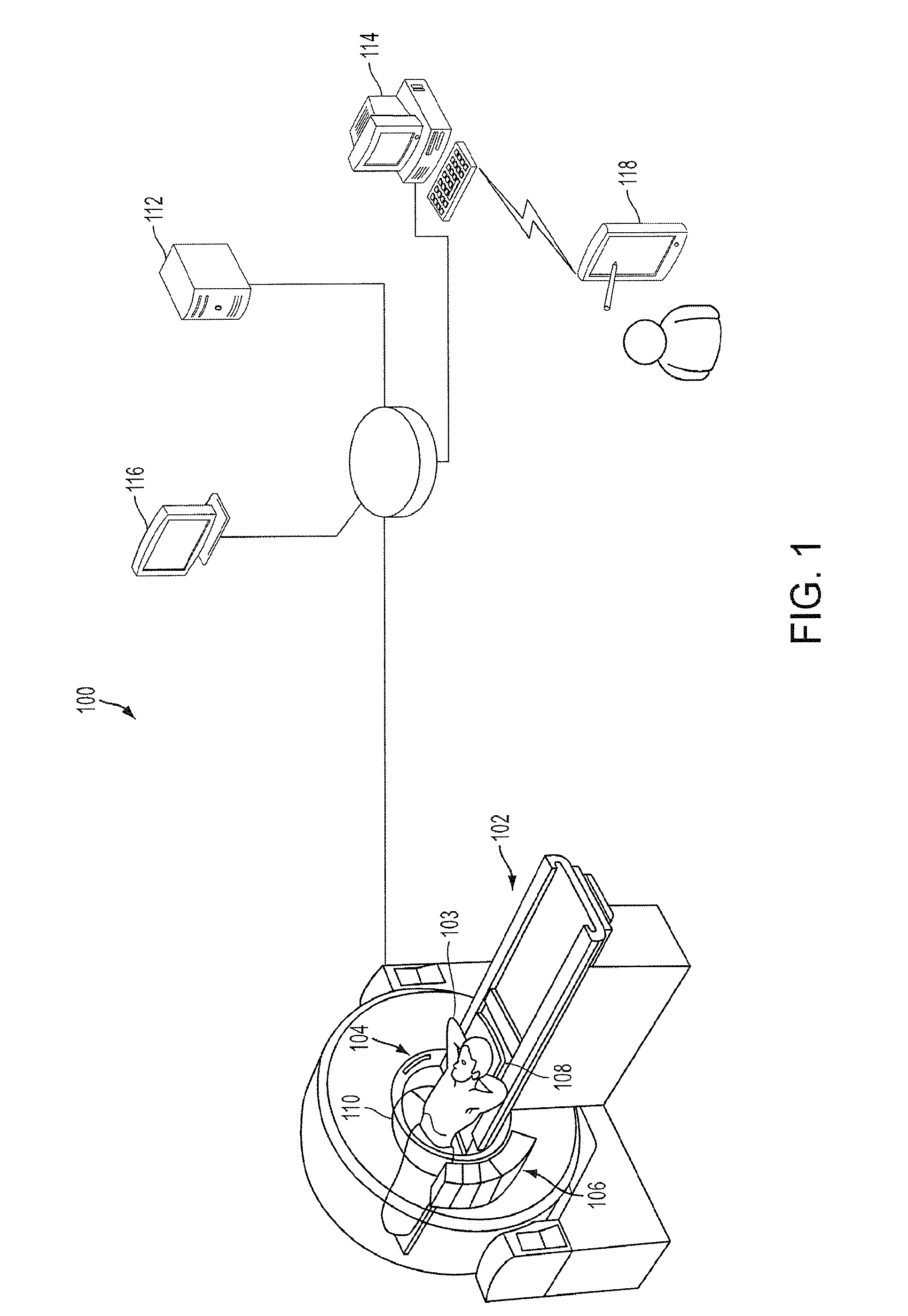
Combined multi-detector ct angiography and ct myocardial perfusion imaging for the diagnosis of coronary artery disease
ActiveUS20110110488A1Material analysis using wave/particle radiationRadiation/particle handlingDiseaseX-ray
A computed tomography system has a support stage constructed and arranged to support a subject while under observation, an x-ray illumination system arranged proximate the support stage to illuminate the subject with x-rays, an x-ray detection system arranged proximate the support stage to detect x-rays after they pass through the subject and to provide signals based on the detected x-rays, and a data processing system in communication with the x-ray detection system to receive the signals from the x-ray detection system. The computed tomography system has a dynamic mode of operation and a scanning mode of operation. The data processing system extracts information concerning a dynamic process of the subject based on signals from both the dynamic mode and the scanning mode of operation.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Myocardial perfusion imaging methods and compositions
A myocardial imaging method that is accomplished by administering one or more adenosine A2A adenosine receptor agonist to a human undergoing myocardial imaging as well as pharmaceutical compositions comprising at least one A2a receptor agonist, at least one liquid carrier, and at least one co-solvent.
Owner:GILEAD SCI INC
X-ray CT apparatus and myocardial perfusion image generating system
ActiveCN1706344AReduce injection volumeReduce X-ray doseComputerised tomographsTomographyCardiac muscleX-ray
The present invention discloses an X-ray CT apparatus (1) for radiating X-rays to a subject (P) for scanning the subject (P) and reconstructing an image inside the subject (P) based on the obtained projection data, It includes an image generation unit and a blood flow image generation unit (24e). The image generating unit generates an image based on the projection data in a state where the concentration of the contrast agent in the myocardial portion of the subject (P) to which the contrast agent is continuously injected can be considered constant. A blood flow image generating unit (24e) generates a blood flow image by removing components of myocardial tissue from a portion of a myocardial region of an image generated by the image generating unit.
Owner:TOSHIBA MEDICAL SYST CORP
Adenosine derivative formulations for medical imaging
A stable composition useful for myocardial perfusion imaging contains one or more 2-alkynyladenosine derivatives; and a solvent which is made up of water and hydroxypropyl-β-cyclodextrin.
Owner:ADENOSINE THERAPEUTICS +1
Myocardial perfusion imaging method
A myocardial imaging method that is accomplished by administering one or more adenosine A2A adenosine receptor agonist to a human undergoing myocardial imaging.
Owner:GILEAD SCI INC
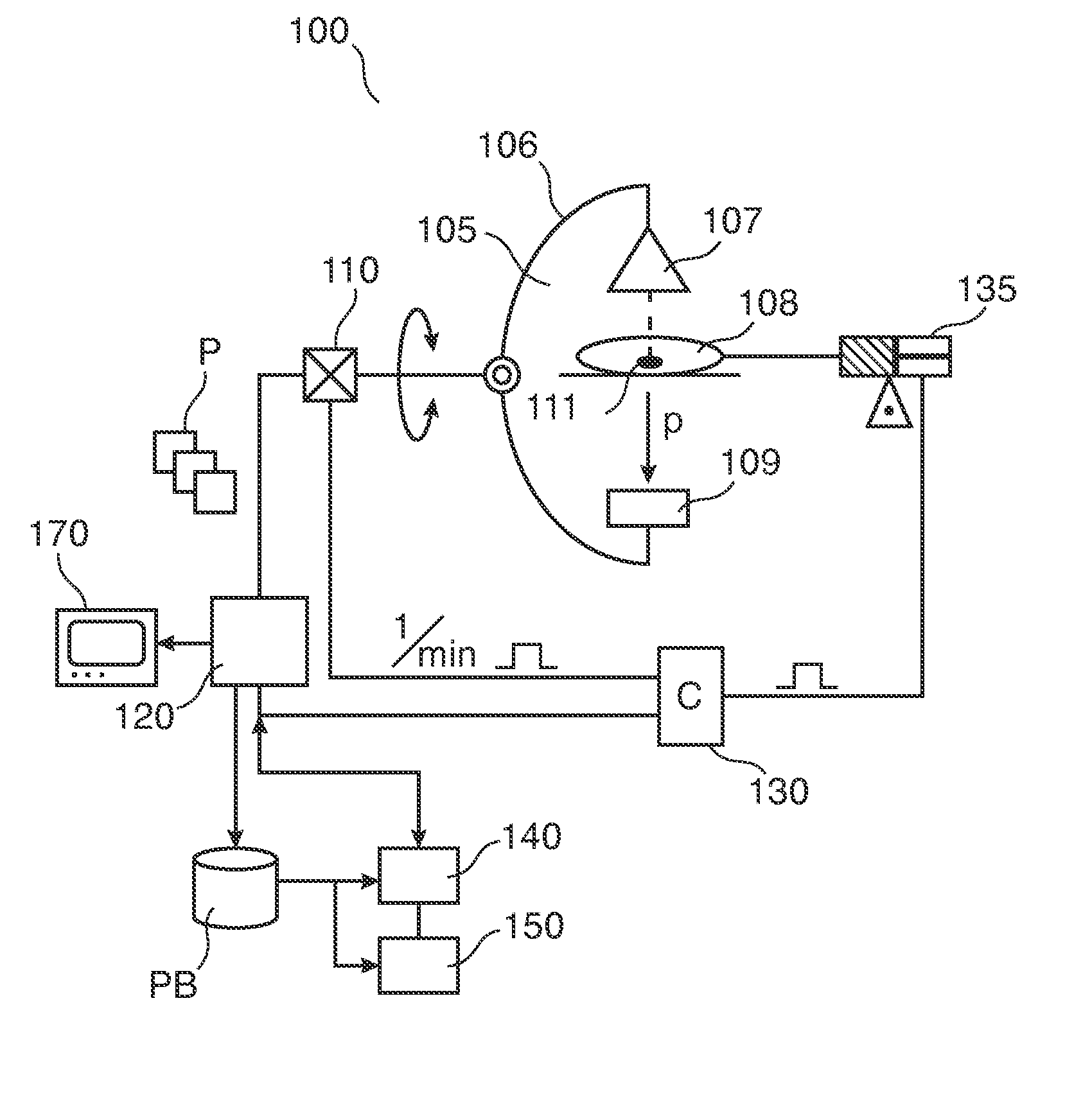
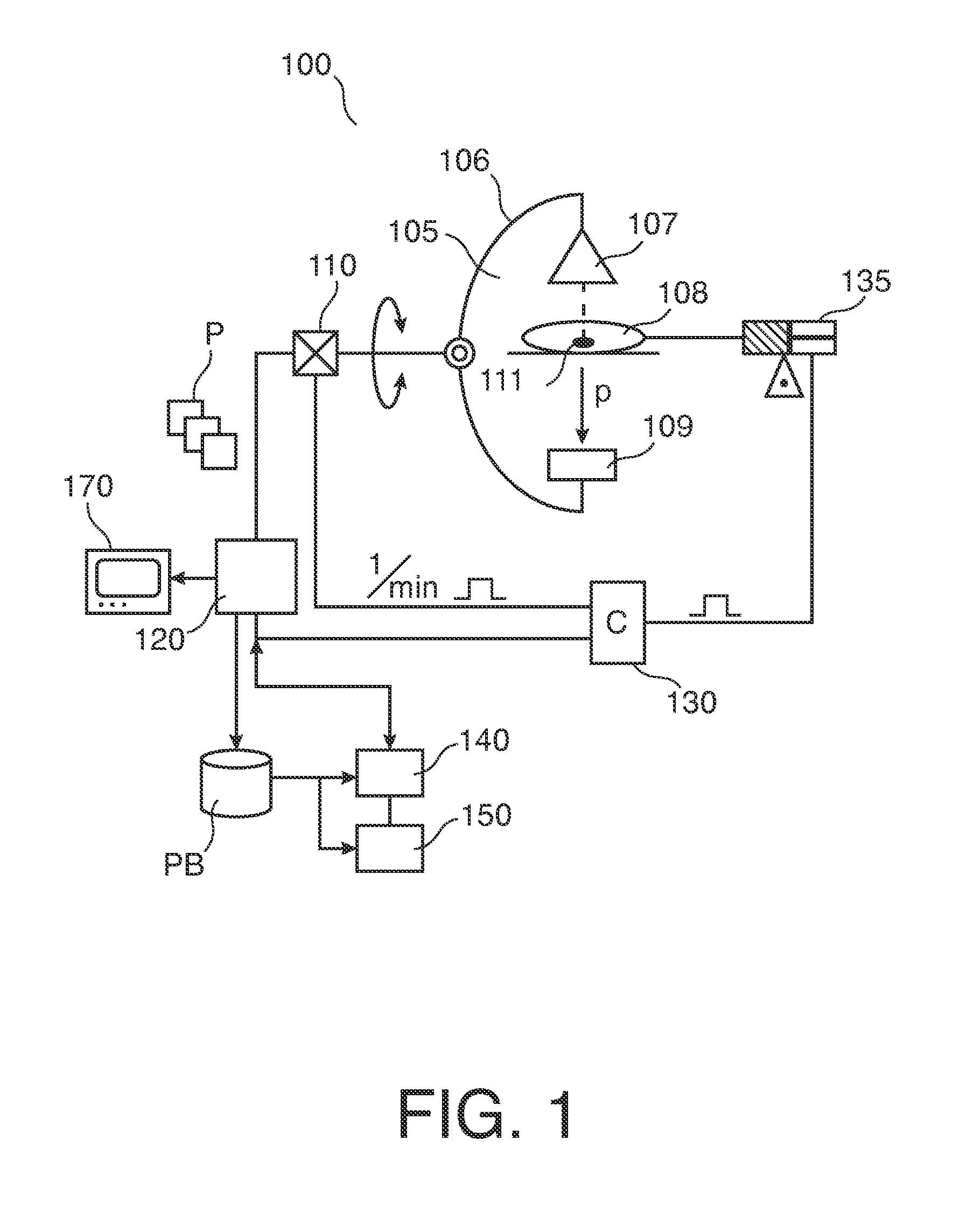
Periodic contrast injections and analysis of harmonics for interventional x-ray perfusion imaging
InactiveUS20150025370A1Reduce signalingSufficient level of accuracyHealth-index calculationTomosynthesisUltrasound attenuationHarmonic
An apparatus (130) and a method for adjusting, in perfusion imaging system, a periodic contrast agent injection rate signal (IS) for an injector (135) as function of an image sampling rate determined by the rotational speed of an X-ray source (107)-detector (109) assembly of an X-ray imager (100). Frequency, periodicity and pulse width of the contrast agent injection rate signal (IS) is adjusted to mitigate temporal signal aliasing in a sample of a time attenuation contrast (TAC) signal.
Owner:KONINKLJIJKE PHILIPS NV
Myocardial perfusion imaging methods and compositions
A myocardial imaging method that is accomplished by administering one or more adenosine A2A adenosine receptor agonist to a human undergoing myocardial imaging as well as pharmaceutical compositions comprising at least one A2a receptor agonist, at least one liquid carrier, and at least one co-solvent.
Owner:GILEAD SCI INC
Novel technetium-99m-labeled higher fatty acid derivative
InactiveCN102311459ALow background uptake performanceReduce fat solubilityIn-vivo radioactive preparationsGroup 7/17 element organic compoundsImaging agentCardiac muscle
The invention relates to a novel technetium-99m-labeled higher fatty acid derivative. The derivative is characterized in that tricarbonyl technetium coordinates with higher fatty acid cyclopentadiene, and the fatty acid connected with cyclopentadiene comprises carbonyl group and unsaturated cyclic group. The derivative can be used as a myocardium imaging agent, and can solve the defects of high liver background or high blood background existing in the prior art when the existing technetium-99mm-labeled higher fatty acid derivative is applied to the myocardium imaging agent; and therefore, the novel technetium-99m-labeled higher fatty acid derivative which has low liver background and low blood background, and can be clinically used as the myocardium imaging agent is provided.
Owner:BEIJING NORMAL UNIVERSITY
Application of berberine or derivatives thereof in preparation of myocardial perfusion imaging agent
The invention provides application of berberine or derivatives thereof in the preparation of a myocardial perfusion imaging agent. The invention further provides application of 18F labelled berberine or derivatives thereof in the preparation of the myocardial perfusion imaging agent. In-vitro researches, in-vivo biological distribution, small animal PED dynamic imaging and the like prove that the 18F labelled berberine derivatives can specifically gather in myocardial cells or heart tissue, has good cardiac muscle targeting distribution features in living animals, are high in heart / surrounding tissue (liver, lung, blood, muscles, bones and the like) comparison value, can be used as a good PET myocardial perfusion imaging agent and is promising in clinical application prospect.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Combined multi-detector CT angiography and CT myocardial perfusion imaging for the diagnosis of coronary artery disease
ActiveUS8615116B2Material analysis using wave/particle radiationRadiation/particle handlingX-rayCoronary heart disease
A computed tomography system has a support stage constructed and arranged to support a subject while under observation, an x-ray illumination system arranged proximate the support stage to illuminate the subject with x-rays, an x-ray detection system arranged proximate the support stage to detect x-rays after they pass through the subject and to provide signals based on the detected x-rays, and a data processing system in communication with the x-ray detection system to receive the signals from the x-ray detection system. The computed tomography system has a dynamic mode of operation and a scanning mode of operation. The data processing system extracts information concerning a dynamic process of the subject based on signals from both the dynamic mode and the scanning mode of operation.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
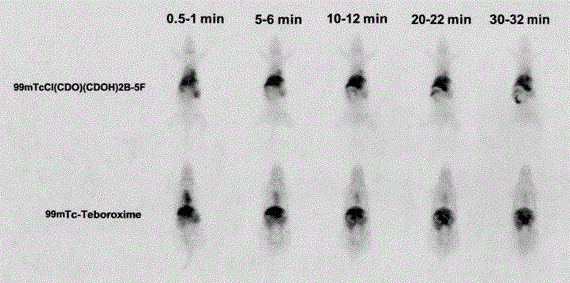
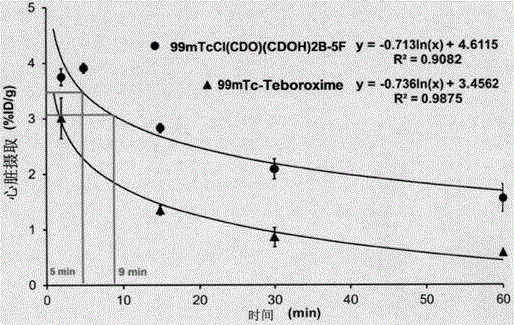
99mTc(III) complex containing arylboronic acid as well as kit formula thereof and application thereof
InactiveCN106749416ALow priceWide variety of sourcesRadioactive preparation carriersIsotope introduction to organic compoundsTechnetium-99mCombinatorial chemistry
The invention provides a 99mTc(III) complex containing arylboronic acid. A molecular formula of the 99mTc(III) complex is 99mTcC1(CDO)(CDOH)2B(F-R), wherein F is furyl and R is methoxyl, formaldehyde or hydroxymethyl. The compound has the characteristics of simple preparation, low price, high labeling rate, high radiochemical purity, high target to non-target ratio, high heart uptake value, long residence time and the like, and can be used as a novel technetium-99m labeled myocardial perfusion imaging agent which is applied to the technical fields of radiopharmaceutical chemistry and clinical nuclear medicines.
Owner:FUWAI HOSPITAL CHINESE ACAD OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE
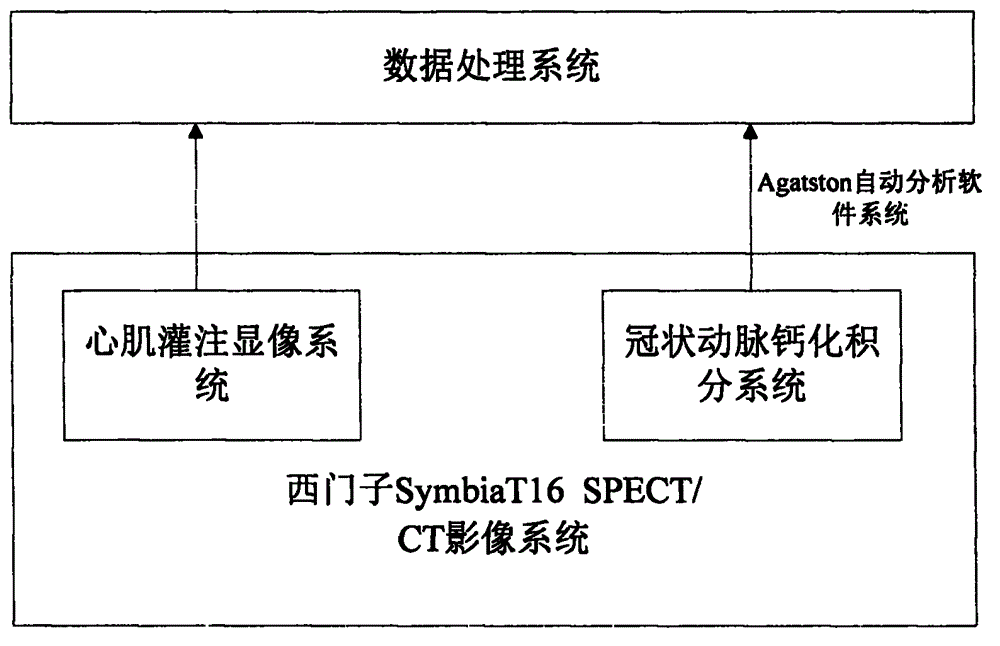
Myocardial perfusion development and coronary artery calcification score combined diagnostic system and method
InactiveCN104783826AShorten the timeShorten diagnostic timeComputerised tomographsTomographyAnalysis dataStatistical analysis
The invention relates to a myocardial perfusion development and coronary artery calcification score combined diagnostic system and method. The diagnostic system comprises a myocardial perfusion development system, a coronary artery calcification score system and a data processing system. Both the myocardial perfusion development system and the coronary artery calcification score system are based on a siemens Symbia T16 SPECT / CT image system, the myocardial perfusion development system is used for collecting nuclein myocardial perfusion development of patients and obtaining diagnostic data by analyzing images. The coronary artery calcification score system works after the myocardial perfusion development system finishes work and is used for collecting coronary artery calcification score data and sending the collected data to an Agatston automatic analysis software system to be computed and analyzed, diagnostic data obtained through the myocardial perfusion development system is combined with the analysis data of the coronary artery calcification score system to be sent to the data processing system to be processed, and the diagnostic result is obtained through statistical analysis. The myocardial perfusion development and coronary artery calcification score combined diagnostic system and method achieve one-stop examination and have the advantages that the system and method are easy and convenient to operate, safe and reliable, the diagnosis time is saved, the cost is saved, the diagnosis efficiency is improved, and the diagnosis accuracy is improved.
Owner:THE FIRST PEOPLES HOSPITAL OF CHANGZHOU
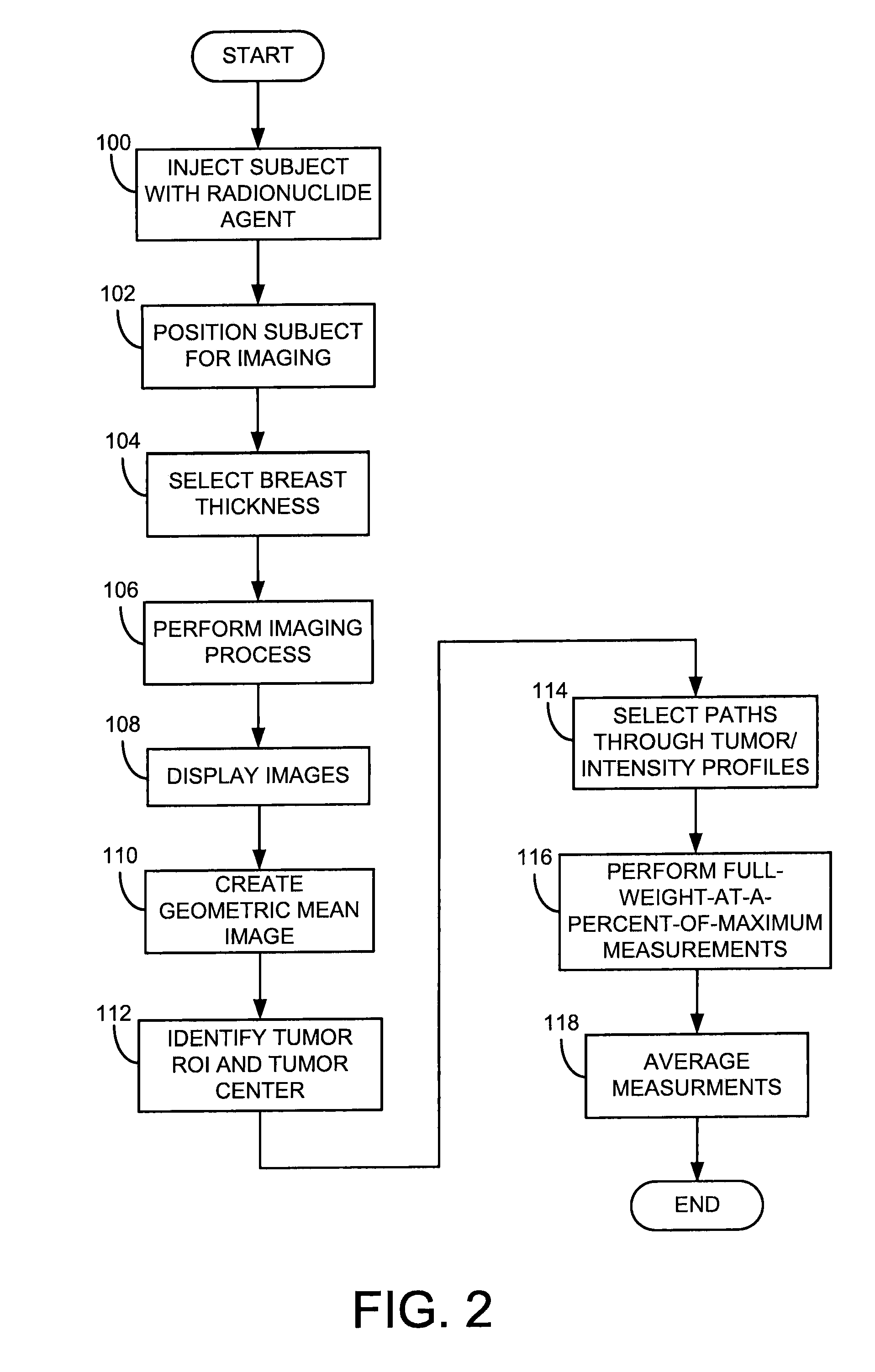
Dual Modality Imaging Of Tissue Using A Radionuclide
A method for imaging a subject includes injecting the subject with a single dose of a radionuclide and acquiring, with a molecular breast imaging (MBI) system, a first set of medical image data of a breast of the subject after injection of the single dose of a radionuclide. The method also includes acquiring, with a myocardial perfusion imaging (MPI) system, a second set of medical image data of a portion of a cardiovascular system of the subject after the single dose of a radionuclide and reconstructing the first set of medical image data into a medical image of the breast of the subject and reconstructing the second set of medical image data into a medical image of the portion of the cardiovascular system of the subject.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Crystal form of regadenoson and preparation method thereof
The present invention relates to the field of medicinal chemistry, and discloses a new crystal form of regadenoson, i.e., a crystal form E of regadenoson, as well as a method for preparing the new crystal form of regadenoson. The crystal form E of regadenoson according to the present invention has excellent performances in terms of radionuclide myocardial perfusion imaging, and has a poor toxicity, good storage stability, and can be used in the preparation of a medicament used as a stress agent for radionuclide myocardial perfusion imaging.
Owner:SHANGHAI ZIYUAN PHARMA
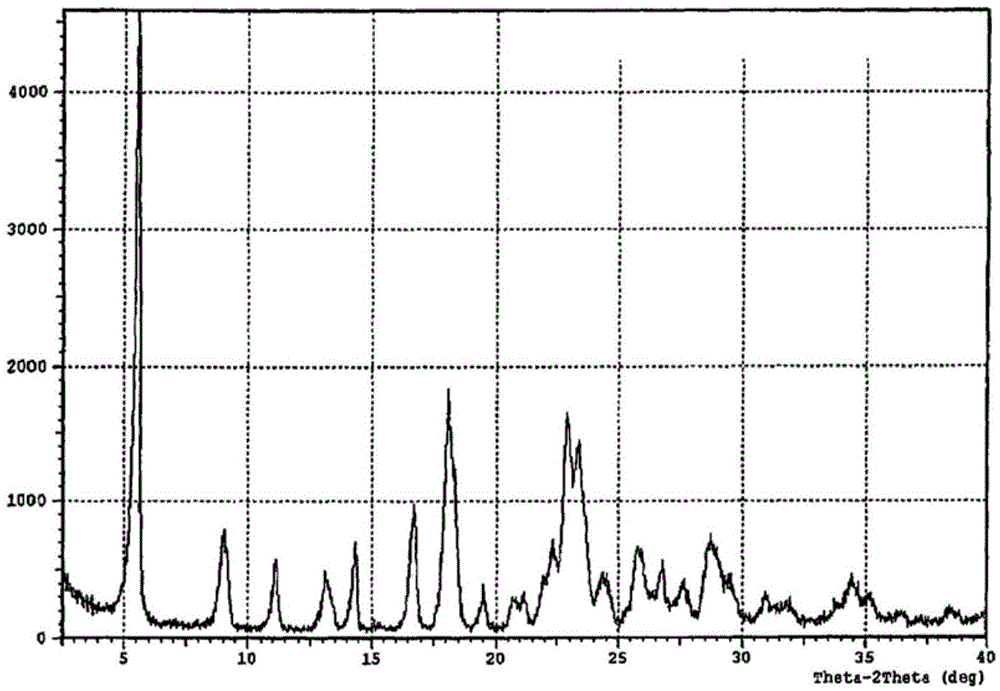
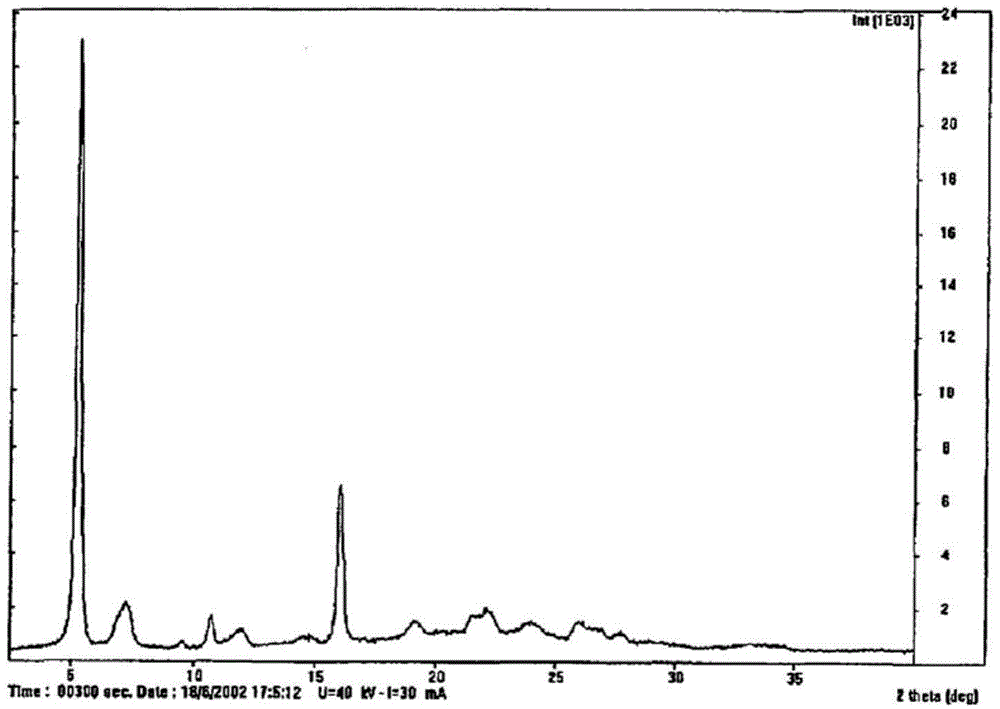
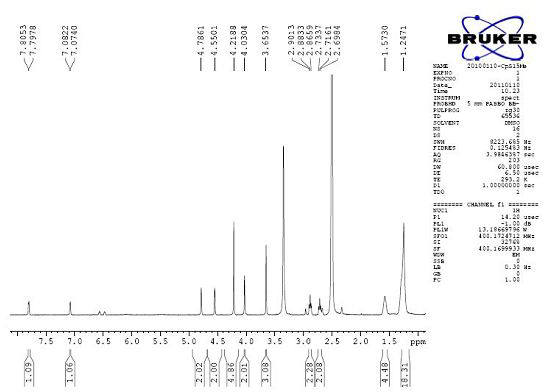
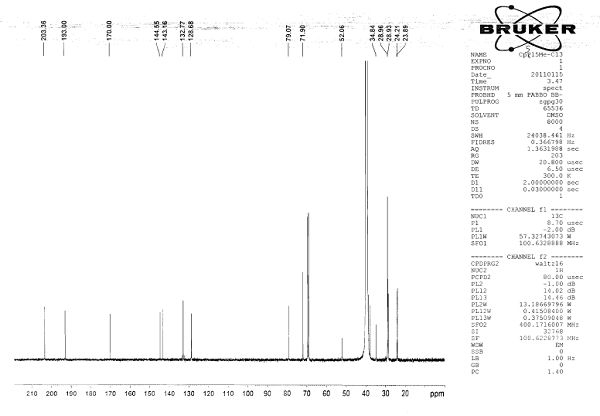
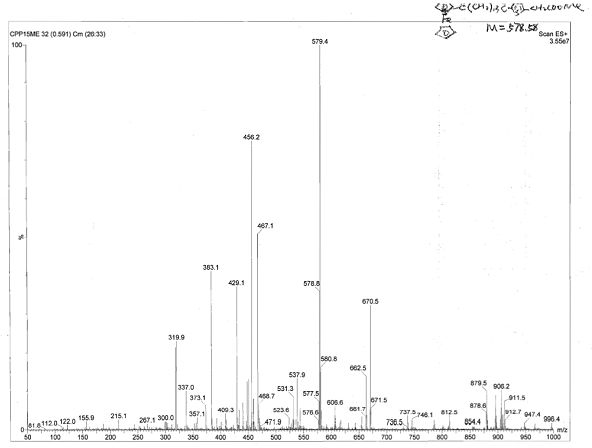
[<18>F]-fluoromethyl triphenylphosphine salt, preparation method and application thereof
InactiveCN105153227AIncrease intakeHigh blood pressure ratioGroup 5/15 element organic compoundsRadioactive preparation carriersTosylhydrazoneDissolution
The invention relates to a [<18>F]-fluoromethyl triphenylphosphine salt, a preparation method and application thereof, and effectively solves the problem of low ratio of myocardial uptake and non-target organs in existing myocardial perfusion imaging agents. The method includes: dissolving silver p-toluenesulfonate in acetonitrile, adding diiodomethane under stirring, conducting heating reflux, performing cooling to room temperature, filtering out light yellow solids, removing the solvent by spinning, and eluting and purifying the residue to obtain bis(p-toluenesulfonyl)-methane; under nitrogen protection, adding a [<18>F] fluoride solution into a K2.2.2 solution and a K2CO3 solution, performing drying, adding acetonitrile, conducting drying again, adding bis(p-toluenesulfonyl)methane and anhydrous acetonitrile into the dried substance, carrying out reaction, and performing cooling to room temperature, conducting column chromatography, adding an acetonitrile solution containing triphenyl phosphine to carry out reaction, and then performing cooling, filtering, column purification, solvent removal, normal saline dissolution and filtering, thus obtaining the [<18>F]-fluoromethyl triphenylphosphine salt. The [<18>F]-fluoromethyl triphenylphosphine salt provided by the invention has the characteristics of high cardiac and myocardial uptake, fast clearance rate in main organs, and high heart blood ratio, heart-lung ratio and heart-liver ratio, low cost, and can acquire clear heart Micro-PET images.
Owner:HENAN UNIV OF CHINESE MEDICINE
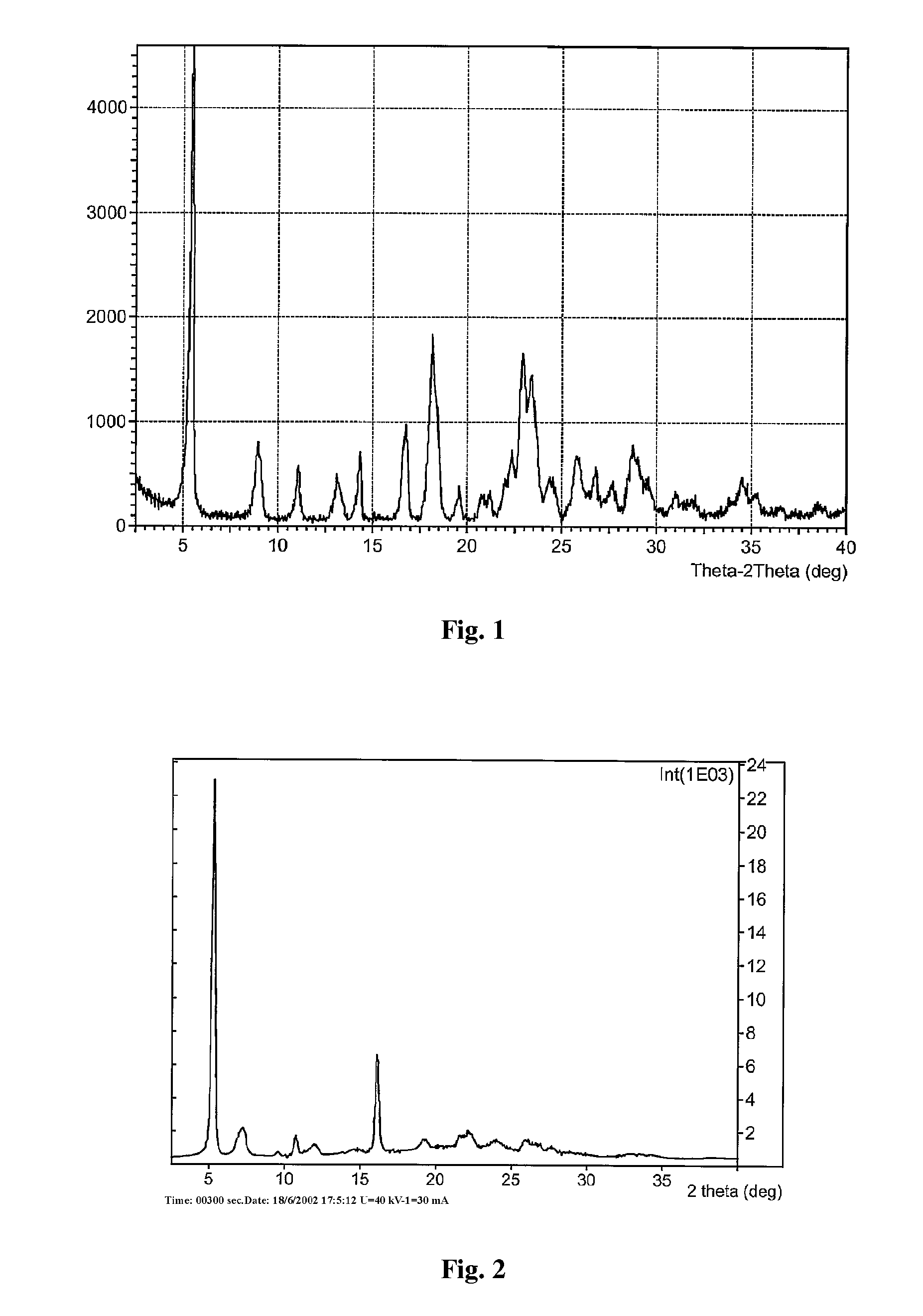
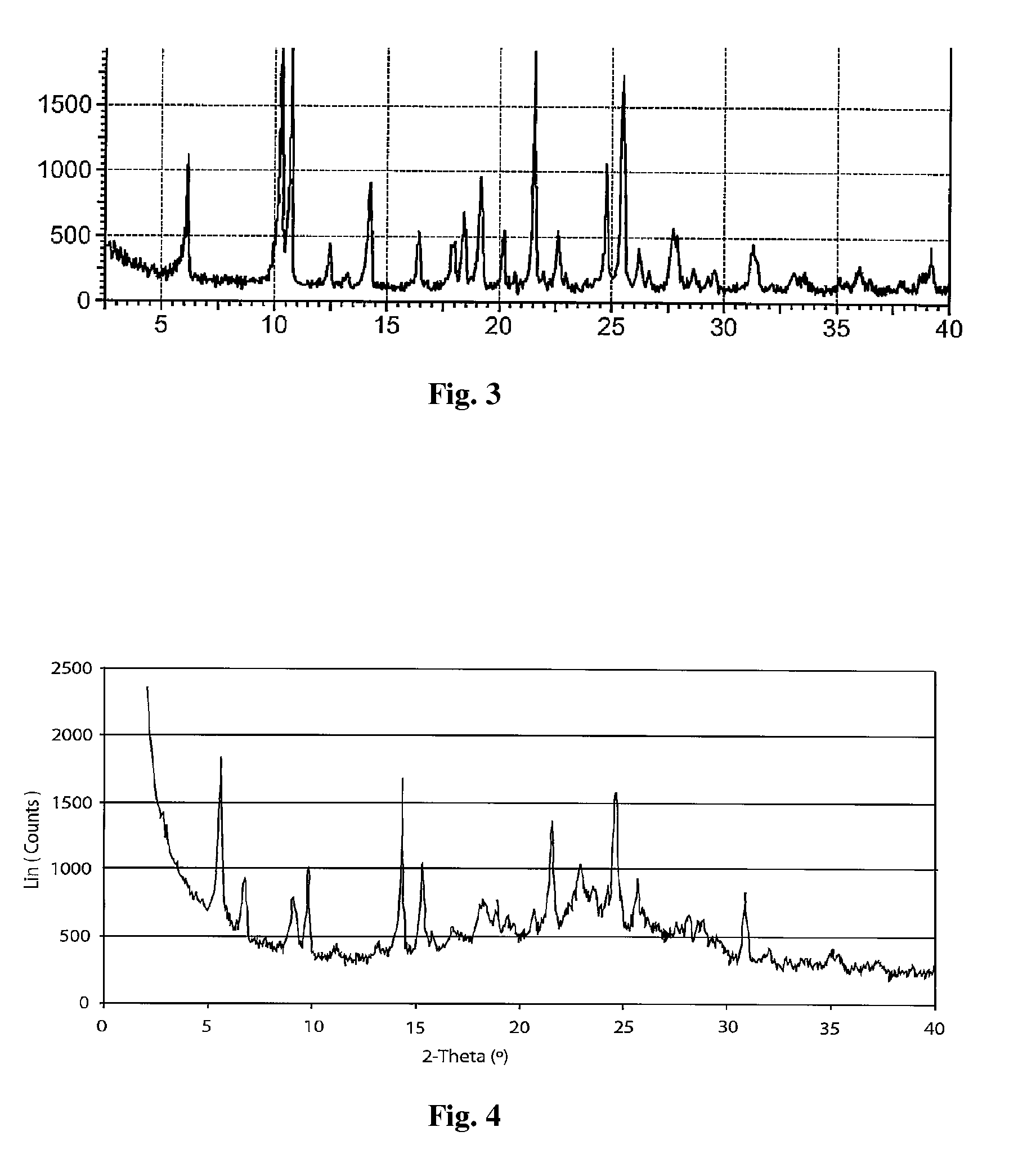
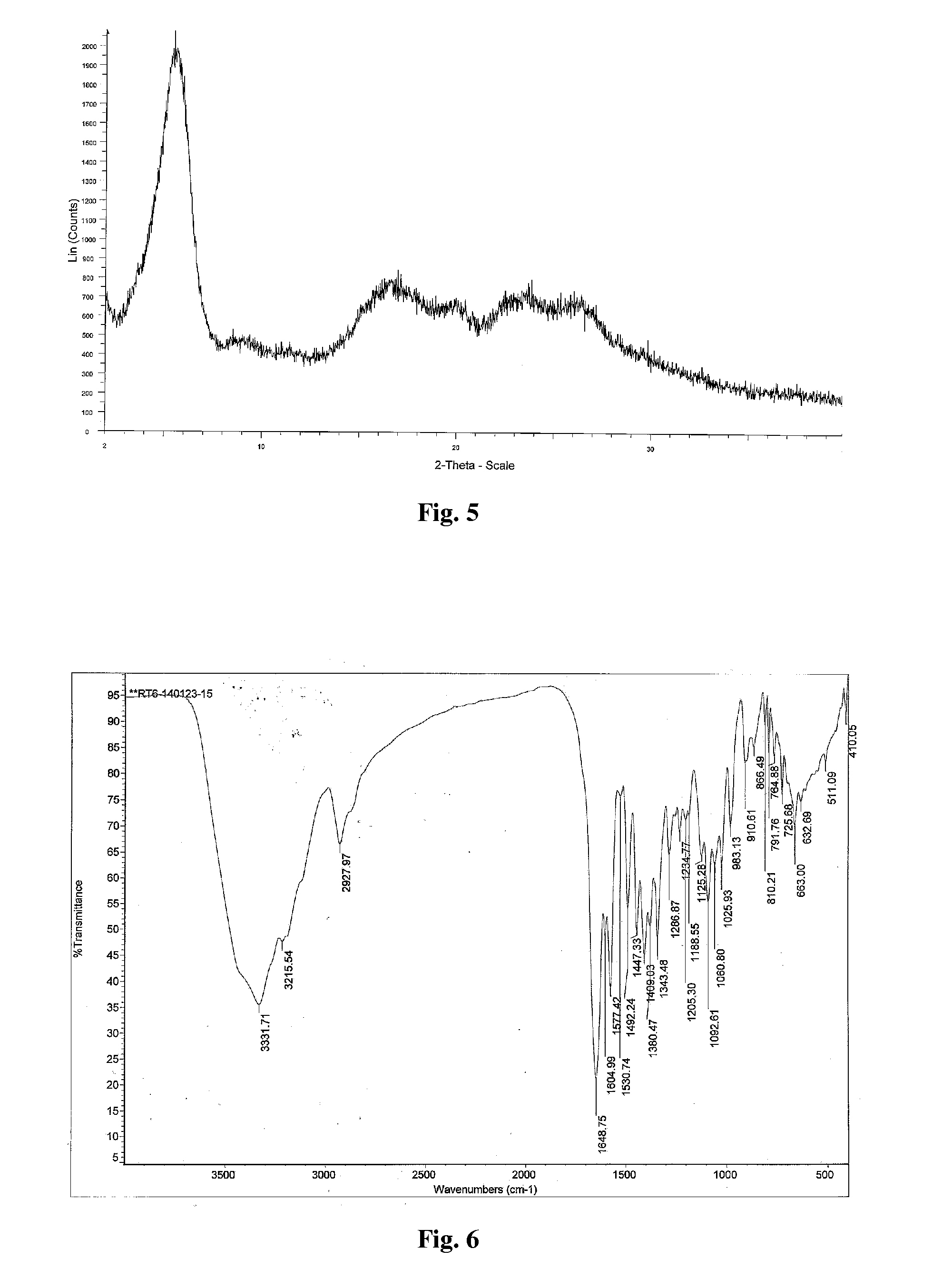
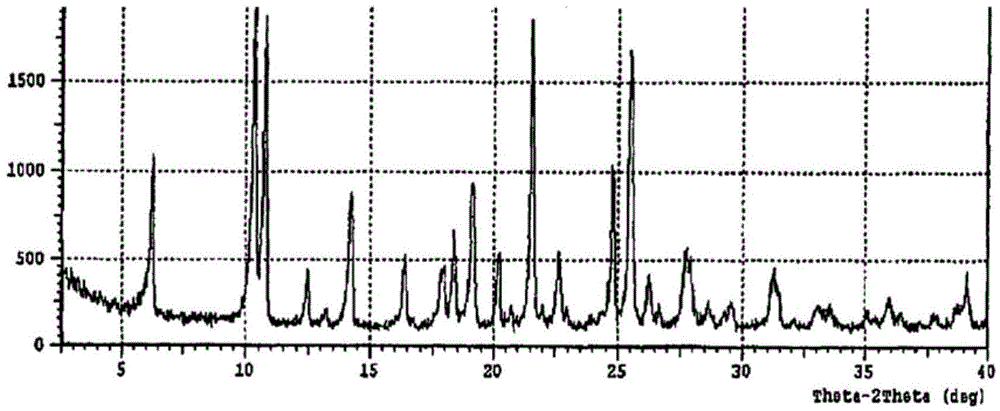
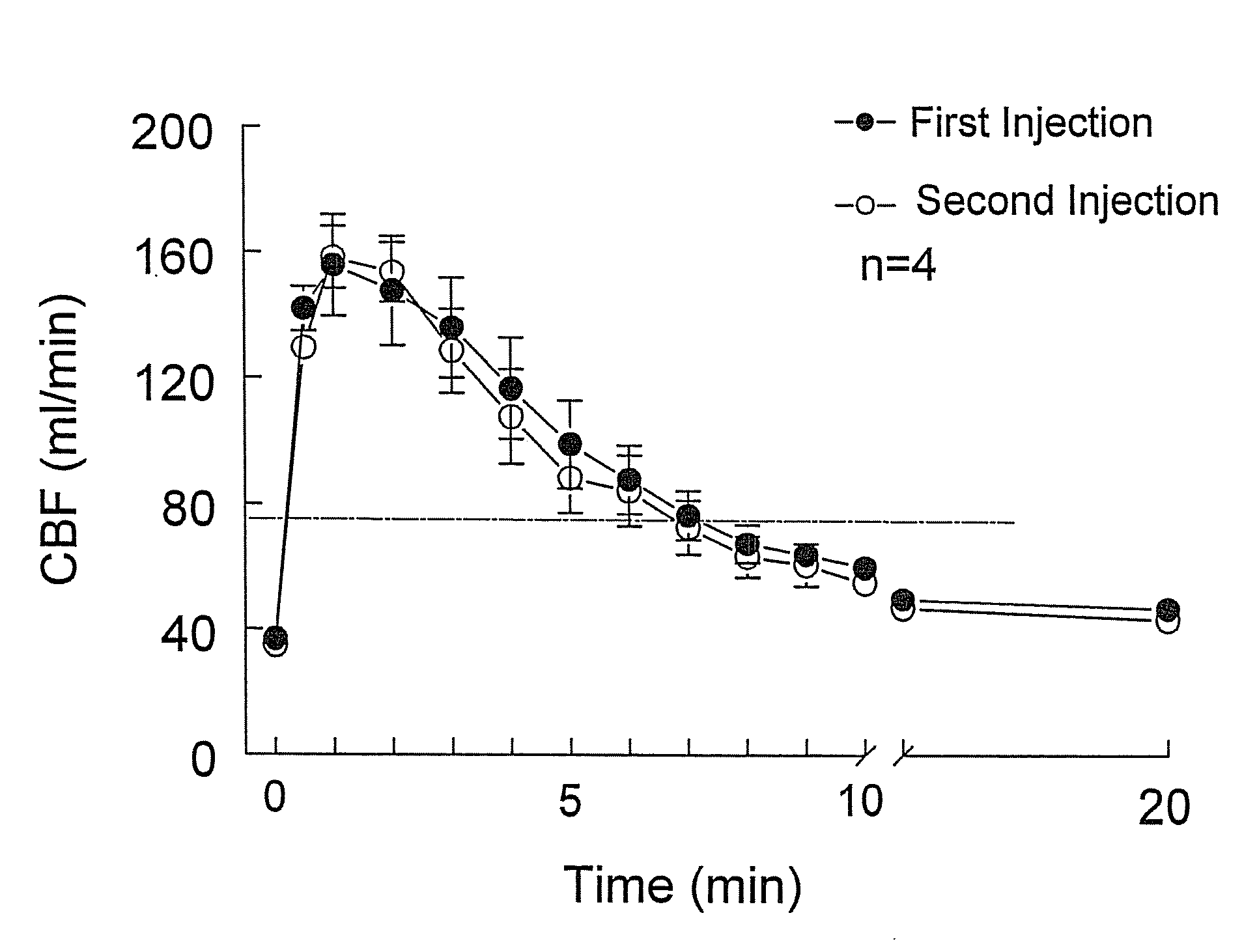
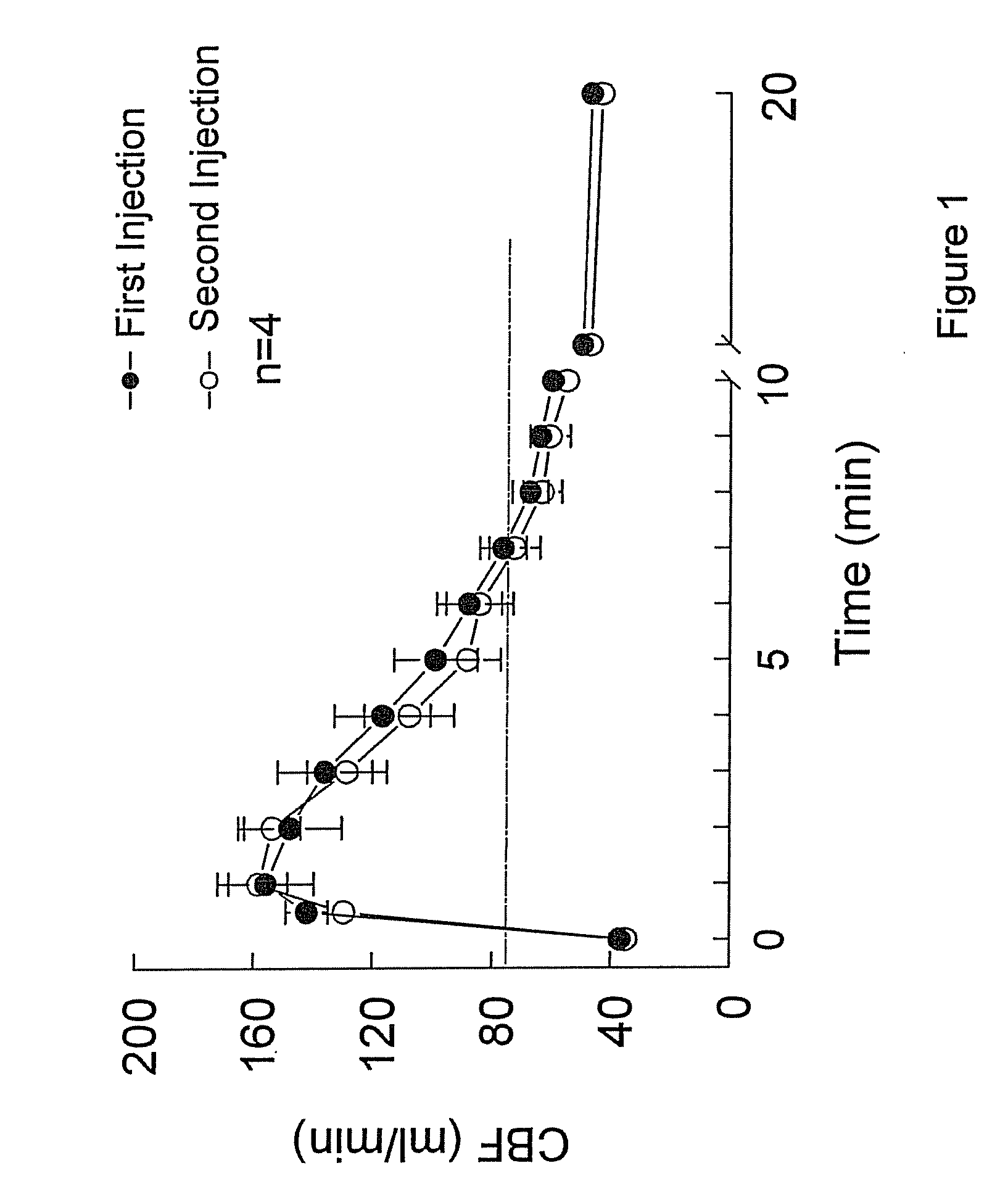
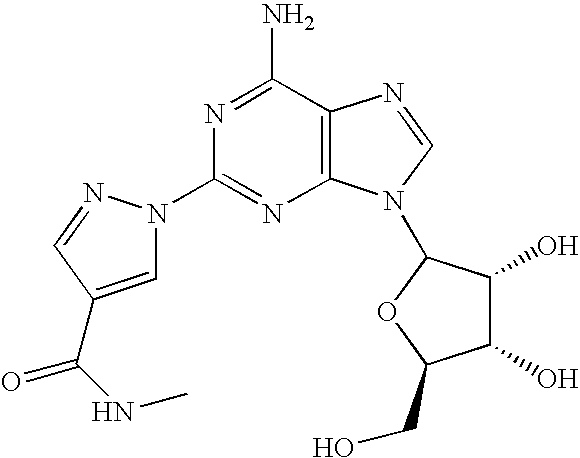
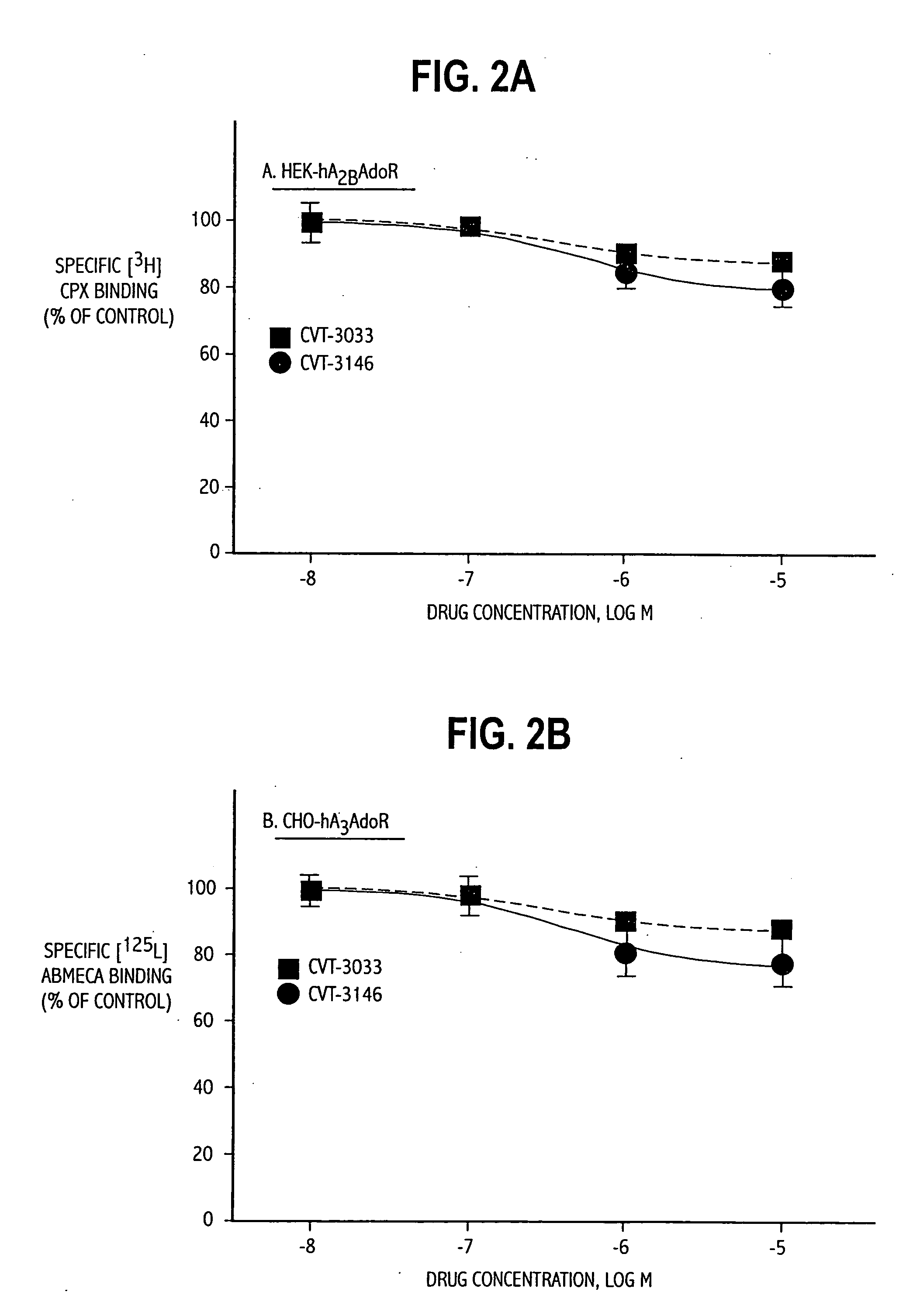
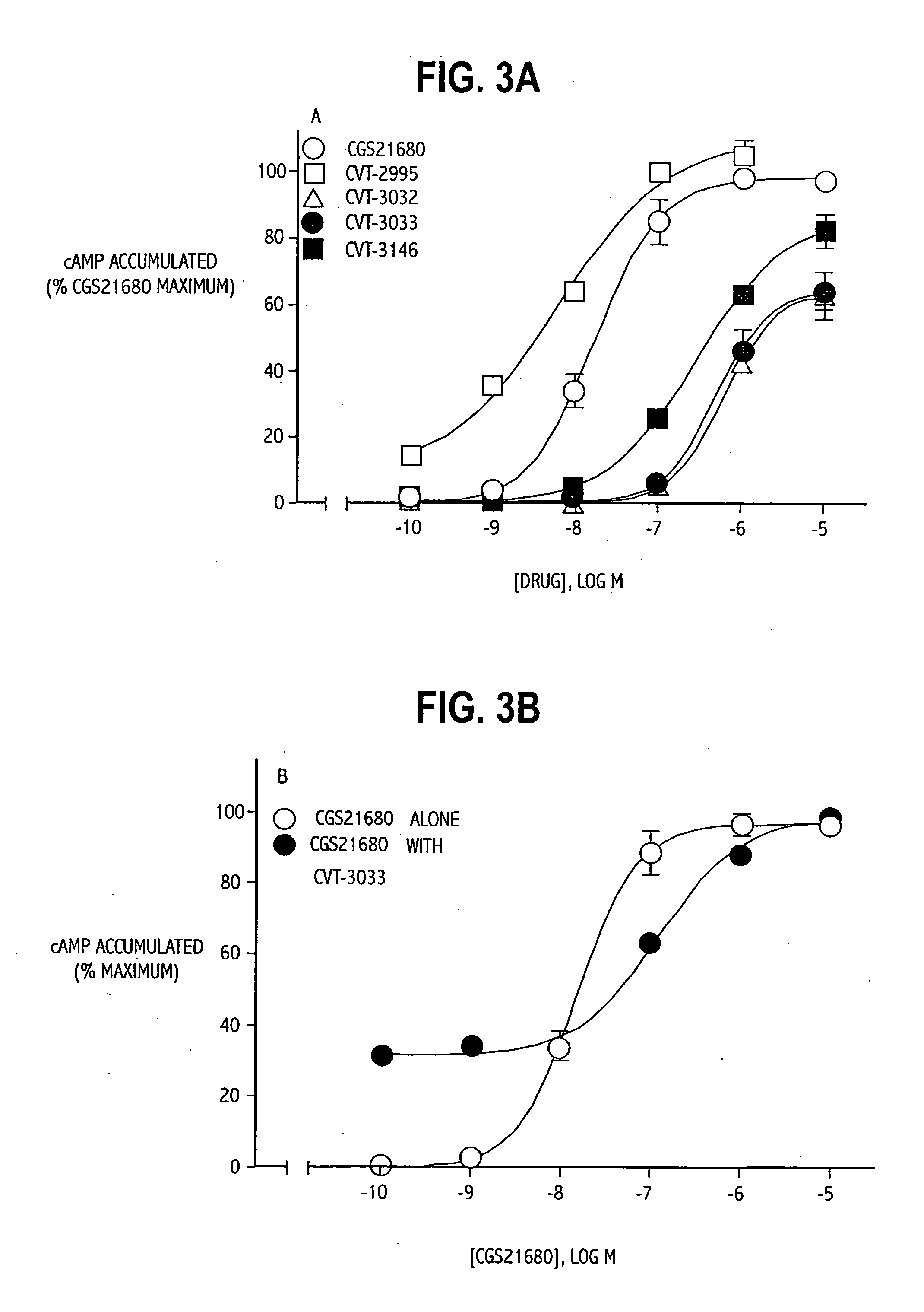
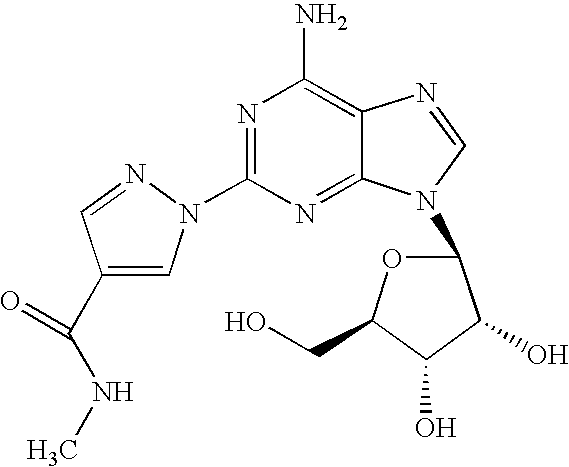
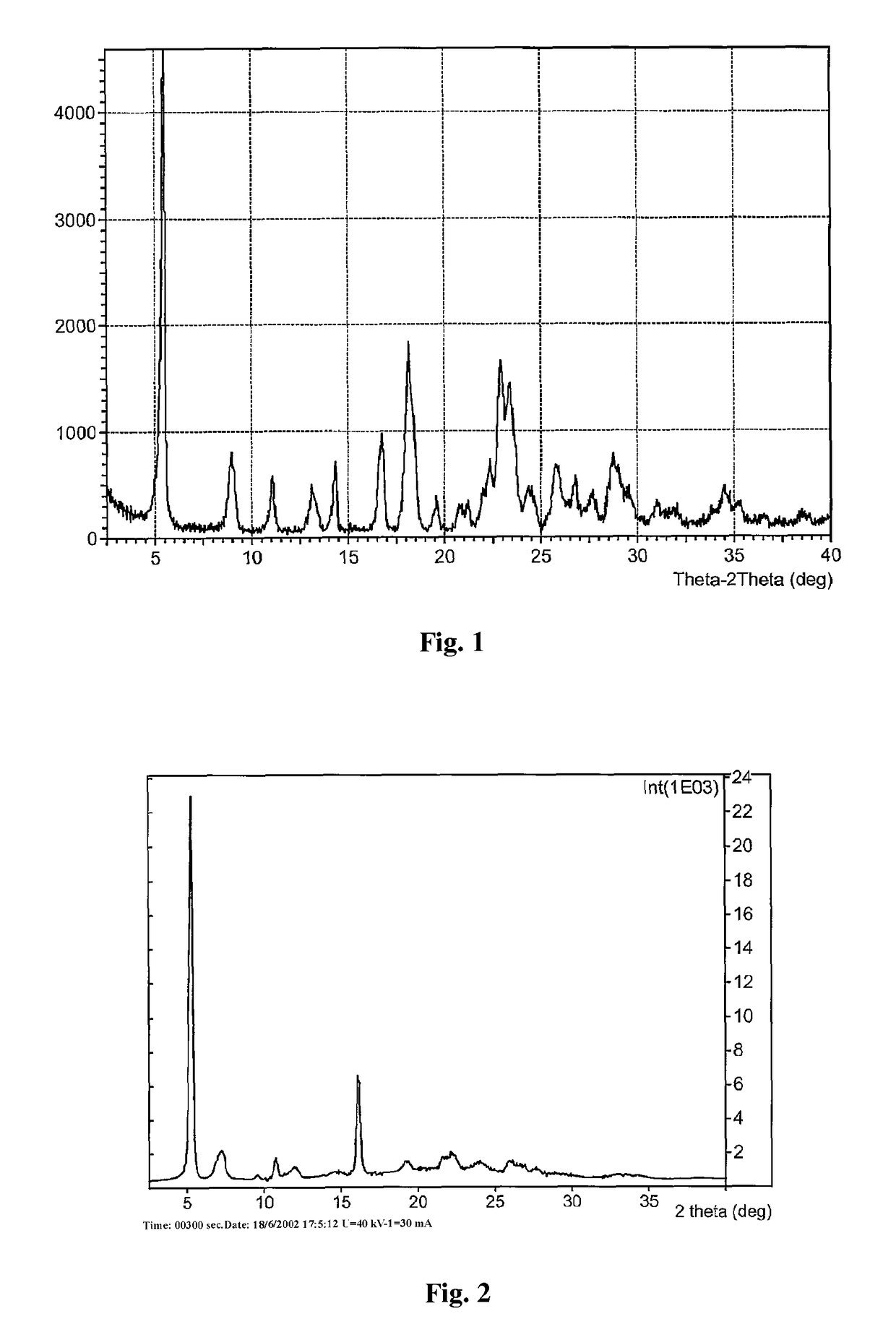
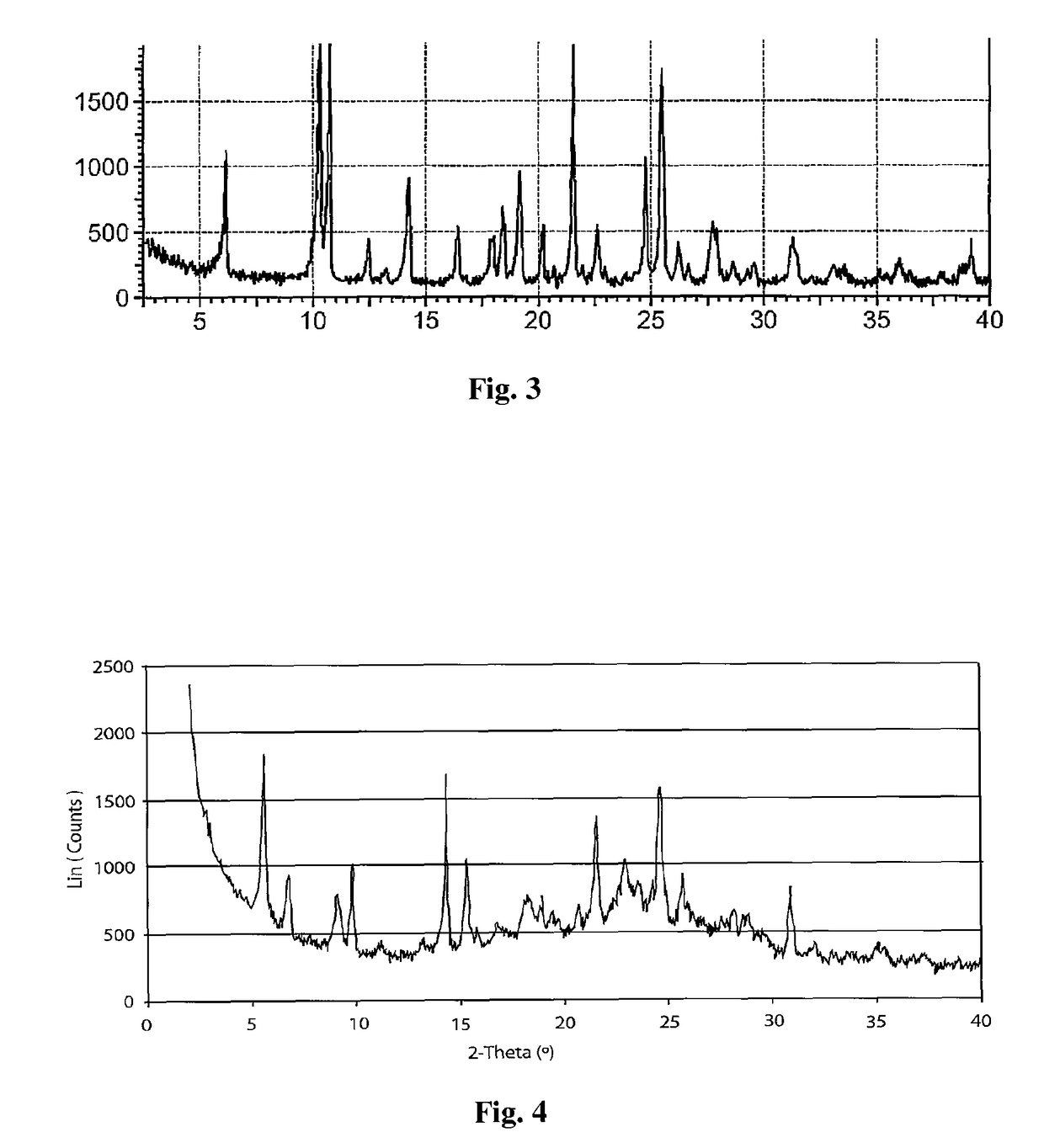
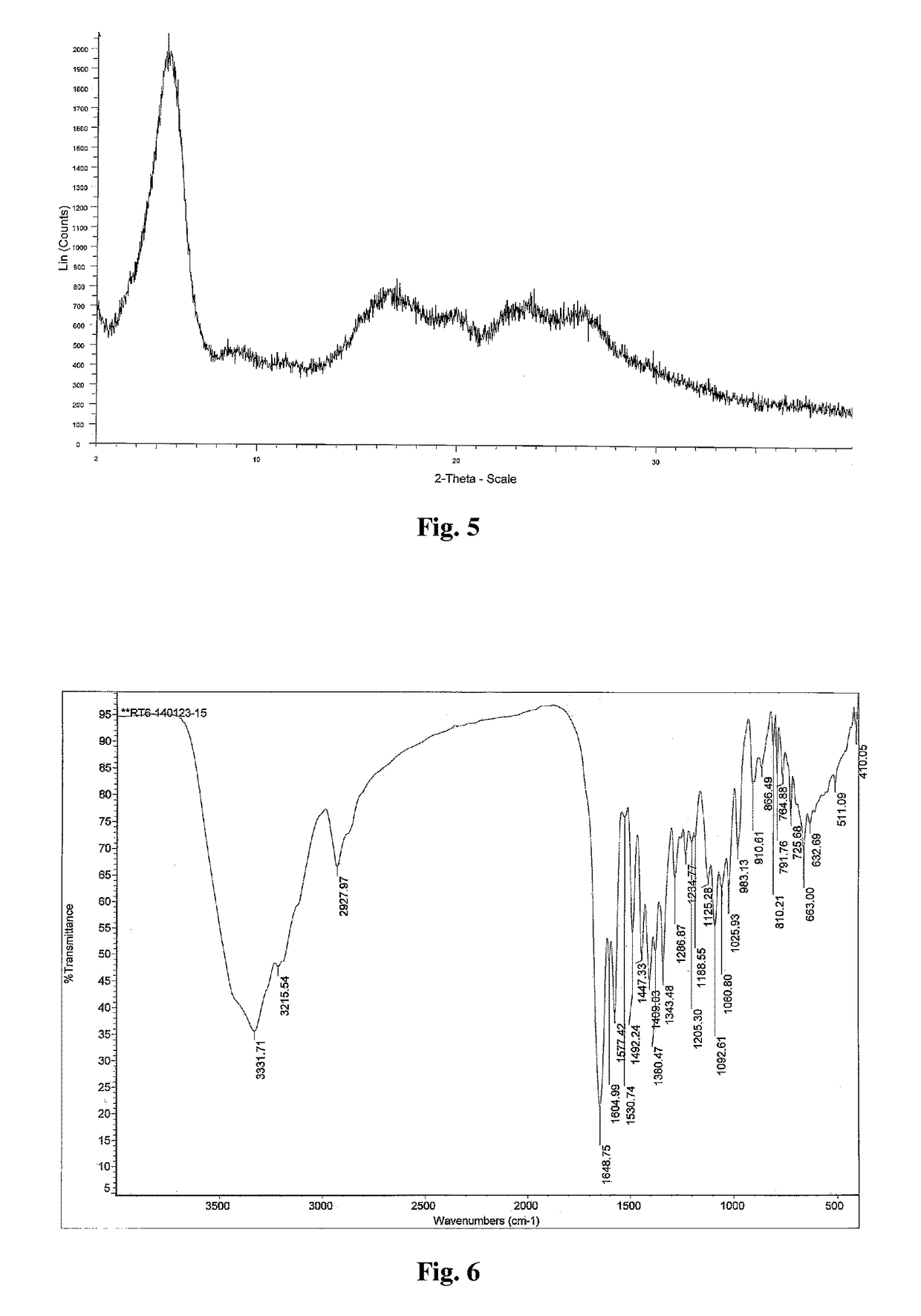
Polymorph of 2-[4-[(methylamino)carbonyl]-1H-pyrazol-1-yl]adenosine
ActiveUS9441006B2Increase capacityReduce formationSugar derivativesSugar derivatives preparationAdenosineX-ray
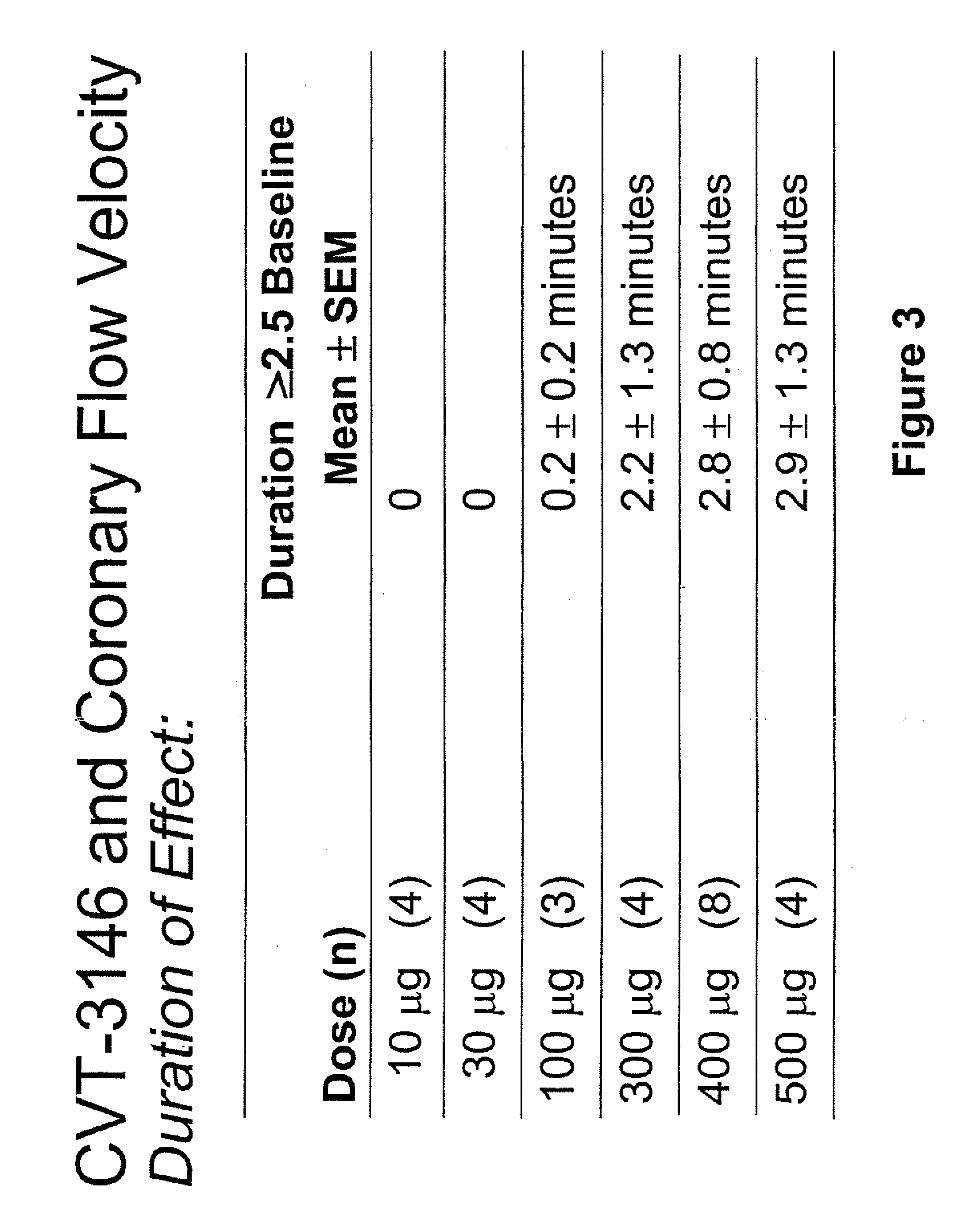
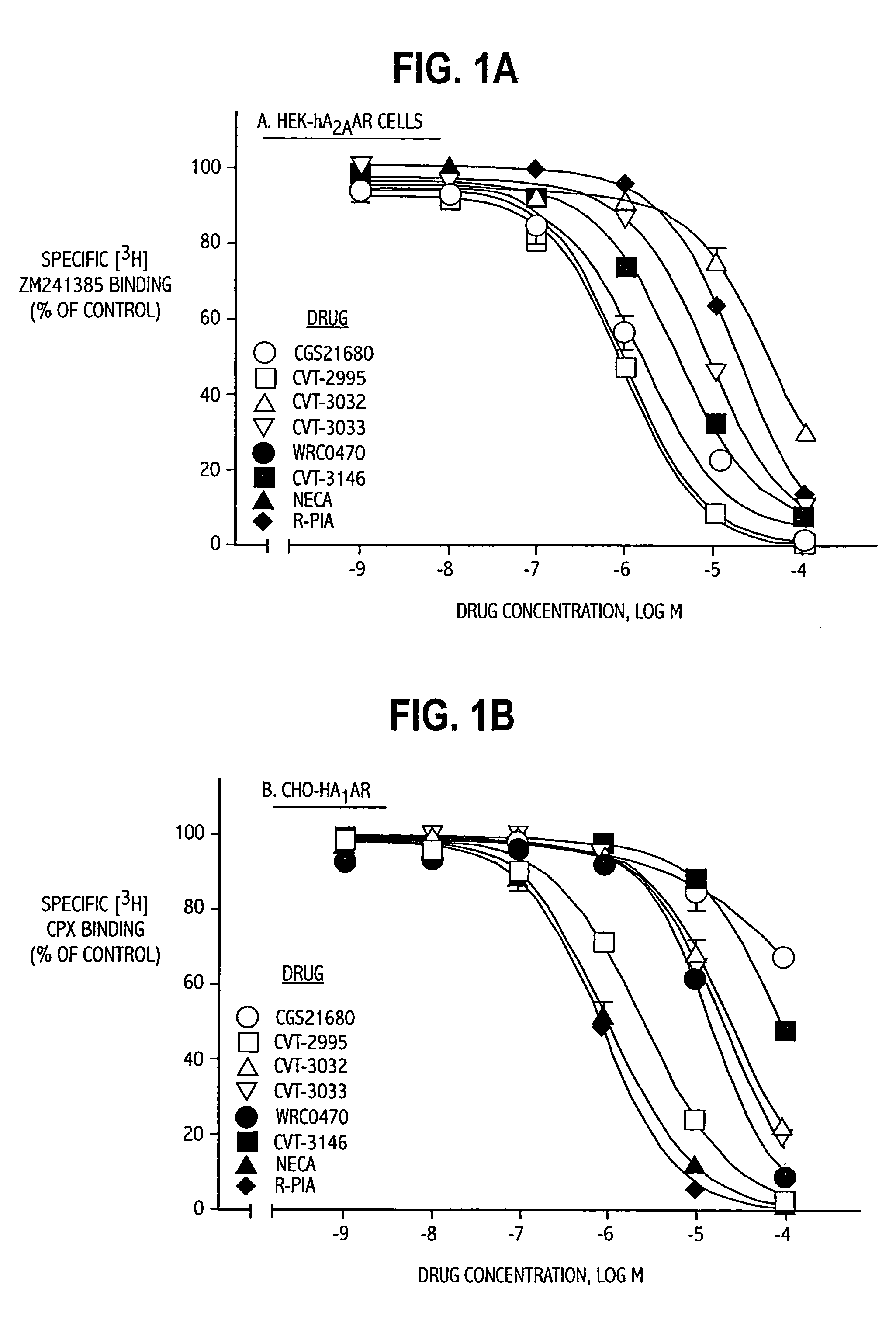
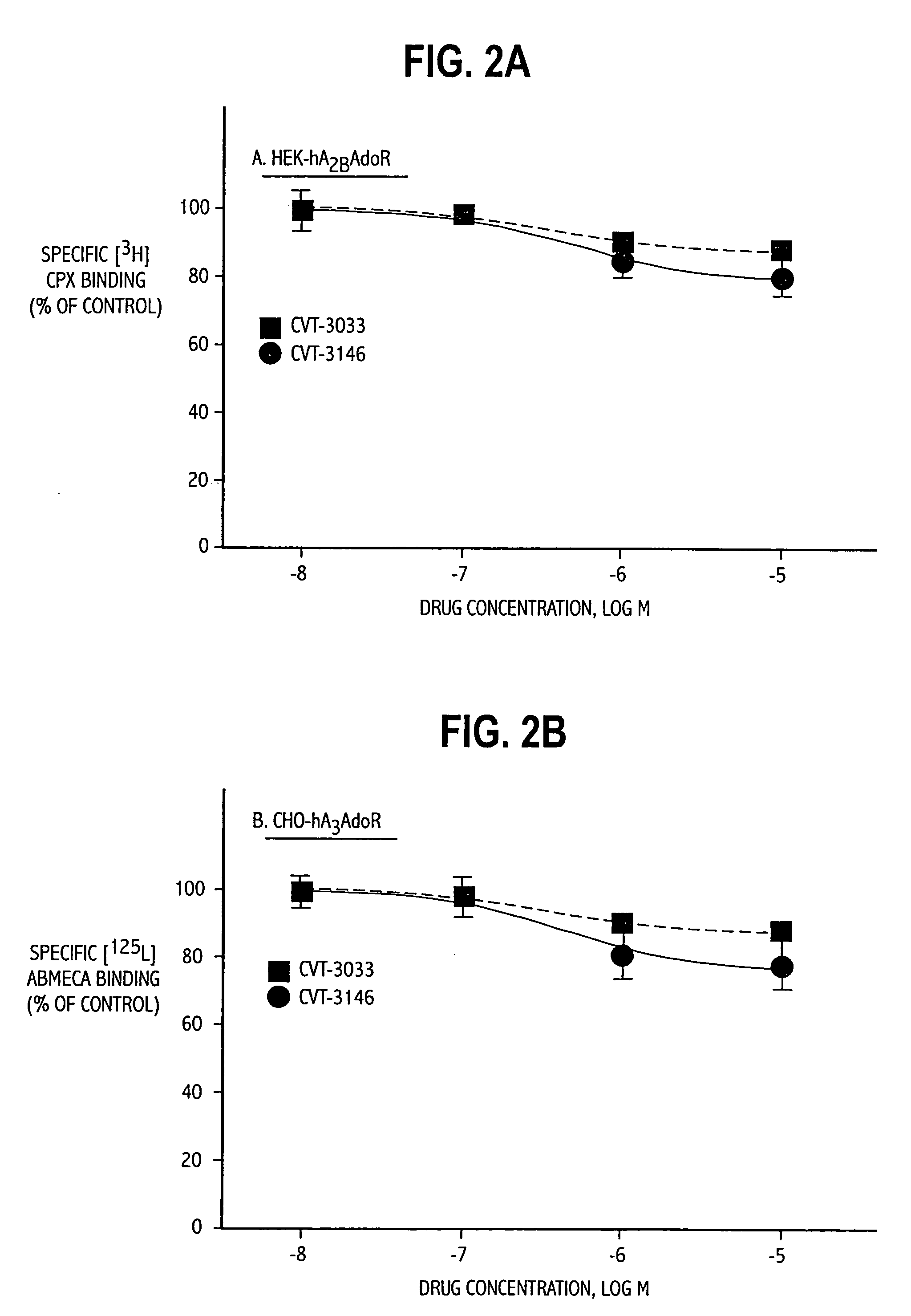
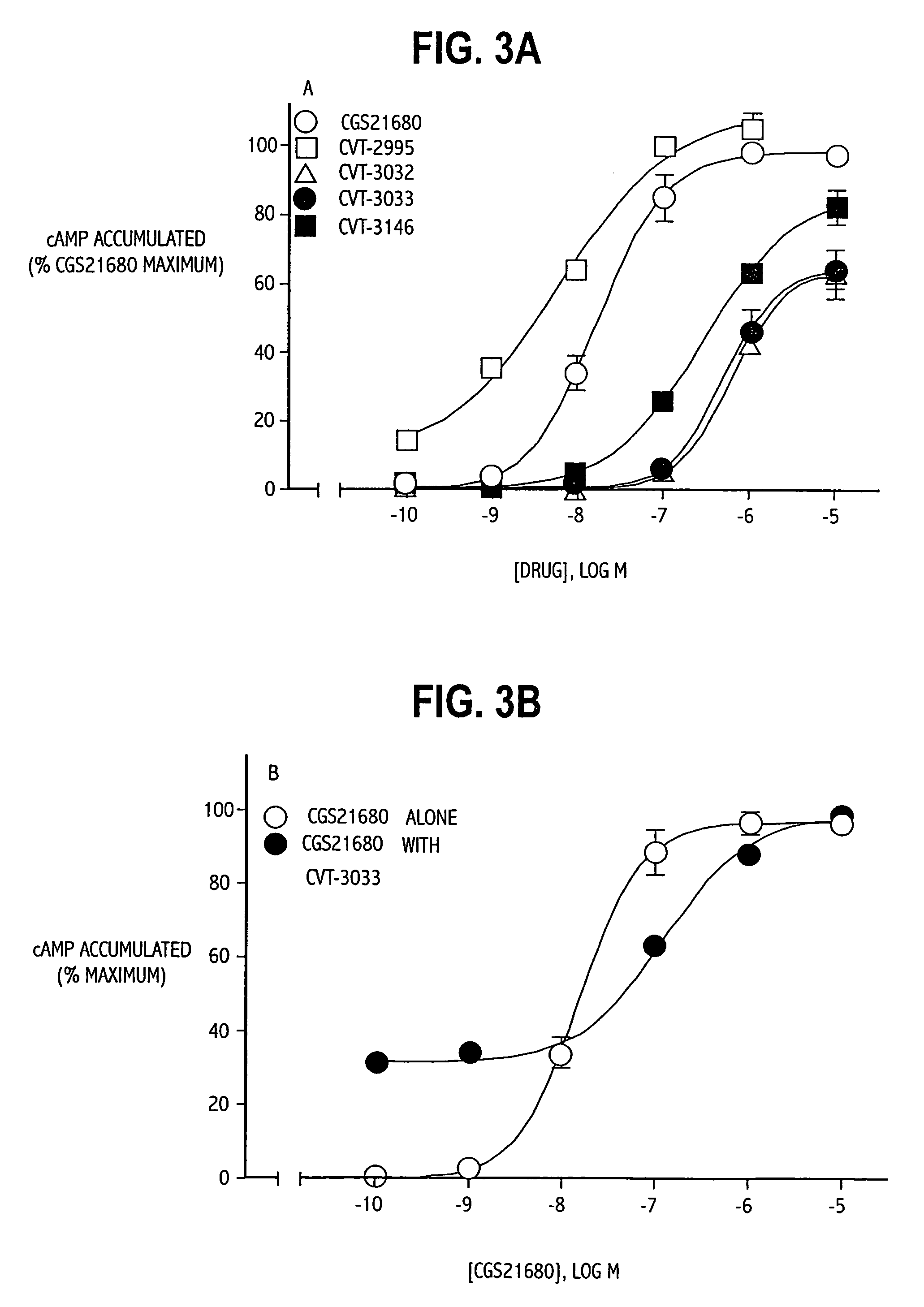
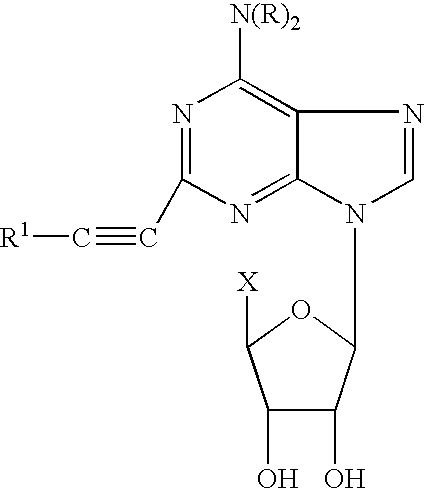
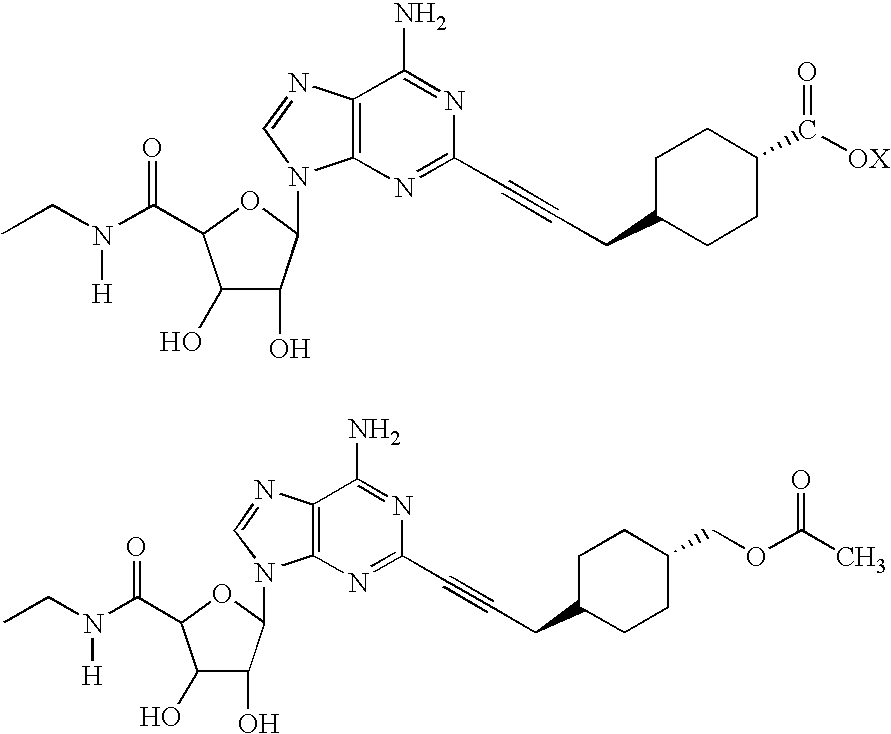
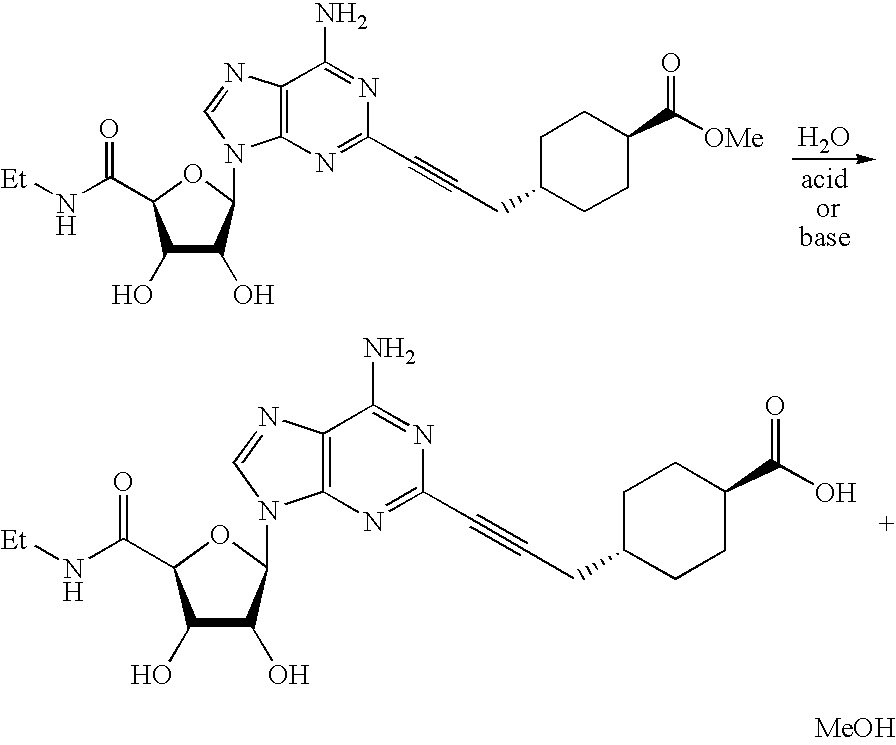
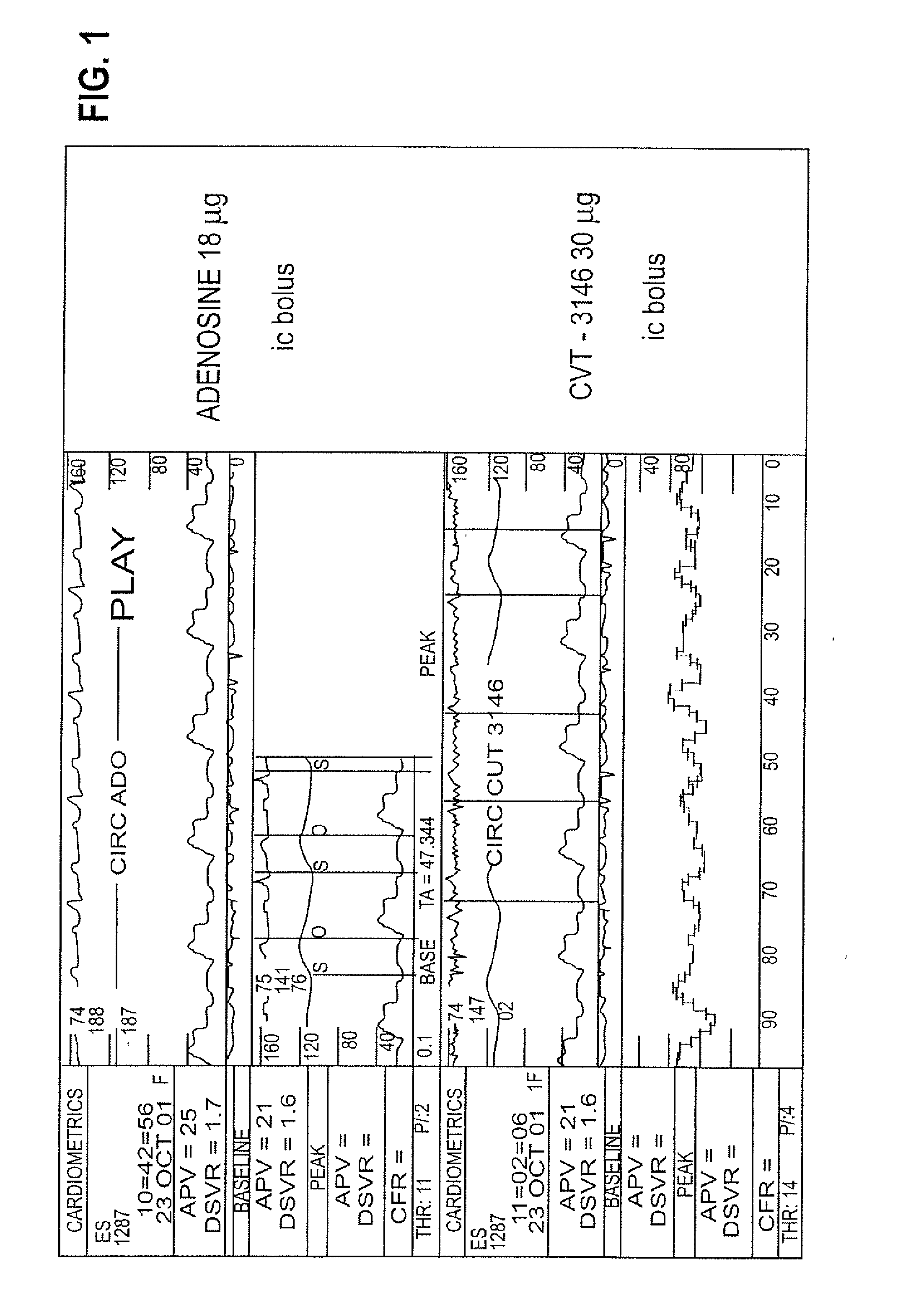
The invention relates to a compound which is, 2-[4-[(methylamino)carbonyl]-1H-pyrazol-1-yl]adenosine, which is a selective A2A adenosine receptor agonist in myocardial imaging. The new polymorph of 2-[4-[(methylamino)carbonyl]-1H-pyrazol-1-yl]adenosine (designated as polymorph E) is characterized by an X-ray diffraction pattern of X-RPD showing the following reflections at 2 Theta=5.8°, 12.3°, 15.9°, 17.3°, 20.5°, 22.6°, 23.6°, 27.7°, and 29.2°; and further characterized by a DSC scan showing marked endotherm in the range of 258 to 264° C.; and further characterized by a specific IR spectra. The invention further relates to a method of preparing the polymorph by recrystallization from other polymorphic forms of 2-[4-[(methylamino)carbonyl]-1H-pyrazol-1-yl]adenosine by a procedure comprising the following operations: mixing of 2-[4-[(methylamino)carbonyl]-1H-pyrazol-1-yl]adenosine with a polar aprotic solvent, preferably with dimethylsulfoxide, and heating to form a saturated solution; cooling of the saturated solution with formation of a turbid solution of 2-[4-[(methylamino)carbonyl]-1H-pyrazol-1-yl]adenosine; addition of the turbid solution of 2-[4-[(methylamino)carbonyl]-1H-pyrazol-1-yl]adenosine to a protic solvent, preferably methanol, with separation of a gel-like precipitate; heating of the separated gel-like precipitate in the protic solvent to a boil with formation of a suspension of polymorph E; and cooling of the suspension, isolation and drying of polymorph E.
Owner:FARMAK
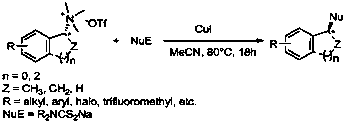
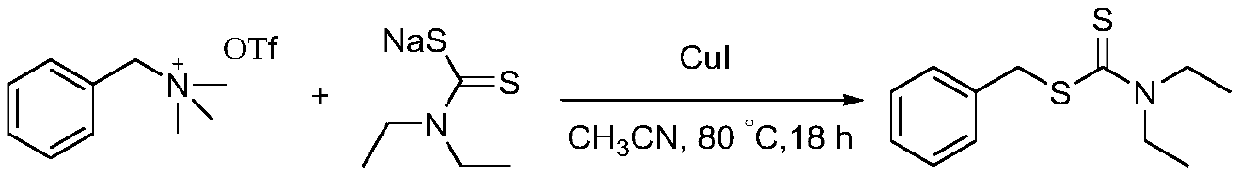
Synthetic method of dialkyl amino dithiocarbamate alkyl ester
In organic sulfur compounds, dialkyl amino dithiocarbamate alkyl ester plays a very important role. Researches show that dialkyl amino dithiocarbamate alkyl ester and the derivatives thereof have widebiological characteristics and pharmacological activity. For example, many studies show that the dialkyl amino dithiocarbamate alkyl ester has various activities, including anti-proliferative, anti-glaucoma, antibacterial, antifungal, breast cancer treatment and cholinesterase inhibition activities, and can be used as a myocardial imaging agent. It is known that dialkyl amino dithiocarbamate alkyl ester and the derivatives thereof can be taken as inhibitors of HIV-1 NCp7, antiviral agents, and non-flavonoid TRPV1 antagonists. Dialkyl amino dithiocarbamate alkyl ester has a wide application range, and is massively produced in the world. The invention provides a method for efficiently synthesizing dialkyl amino dithiocarbamate alkyl ester. Under the catalysis of CuI, a chiral quaternary ammonium salt and a dialkyl amino dithioformate are used for C-S cross coupling for the first time, and dialkyl amino dithiocarbamate alkyl ester is prepared. The target product is further converted andcoupled with brominated aromatic hydrocarbons to obtain high-purity enantiomer chiral thioethers. The synthesis process has the advantages of mild reaction conditions, wide universality, no toxicity or danger, high yield, wide substrate range and the like.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Crystal form of regadenoson and preparation method thereof
ActiveUS20170002036A1Improve stabilityImprove performanceSugar derivativesOrganic chemistry methodsMedicineRegadenoson
The present invention relates to the field of medicinal chemistry, and discloses a new crystal form of regadenoson, i.e., a crystal form E of regadenoson, as well as a method for preparing the new crystal form of regadenoson. The crystal form E of regadenoson according to the present invention has excellent performances in terms of radionuclide myocardial perfusion imaging, and has a poor toxicity, good storage stability, and can be used in the preparation of a medicament used as a stress agent for radionuclide myocardial perfusion imaging.
Owner:SHANGHAI ZIYUAN PHARMA
Regadenoson crystal form and preparation method thereof
InactiveCN105085593AImprove performanceWeak toxicityOrganic active ingredientsSugar derivativesRegadenosonMedicinal chemistry
The present invention relates to the field of pharmaceutical chemistry, and discloses a new regadenoson crystal form, ie., regadenoson crystal form E, and a preparation method of the new regadenoson crystal form. According to the present invention, the regadenoson crystal form E has excellent performances in the field of radionuclide myocardial perfusion imaging, has characteristics of weak toxicity and good storage stability, and can be used for preparing drugs for radionuclide myocardial perfusion imaging stress agents.
Owner:SHANGHAI ZIYUAN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![[<18>F]-fluoromethyl triphenylphosphine salt, preparation method and application thereof [<18>F]-fluoromethyl triphenylphosphine salt, preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/ae30ed45-e638-44cd-b4e0-5748d7750814/150704100344.PNG)
![[<18>F]-fluoromethyl triphenylphosphine salt, preparation method and application thereof [<18>F]-fluoromethyl triphenylphosphine salt, preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/ae30ed45-e638-44cd-b4e0-5748d7750814/150704100346.PNG)
![[<18>F]-fluoromethyl triphenylphosphine salt, preparation method and application thereof [<18>F]-fluoromethyl triphenylphosphine salt, preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/ae30ed45-e638-44cd-b4e0-5748d7750814/150704100350.PNG)
![Polymorph of 2-[4-[(methylamino)carbonyl]-1<i>H</i>-pyrazol-1-yl]adenosine Polymorph of 2-[4-[(methylamino)carbonyl]-1<i>H</i>-pyrazol-1-yl]adenosine](https://images-eureka.patsnap.com/patent_img/e49ee01a-c3fa-40ac-b011-1d96cff35528/US09441006-20160913-D00001.PNG)
![Polymorph of 2-[4-[(methylamino)carbonyl]-1<i>H</i>-pyrazol-1-yl]adenosine Polymorph of 2-[4-[(methylamino)carbonyl]-1<i>H</i>-pyrazol-1-yl]adenosine](https://images-eureka.patsnap.com/patent_img/e49ee01a-c3fa-40ac-b011-1d96cff35528/US09441006-20160913-D00002.PNG)
![Polymorph of 2-[4-[(methylamino)carbonyl]-1<i>H</i>-pyrazol-1-yl]adenosine Polymorph of 2-[4-[(methylamino)carbonyl]-1<i>H</i>-pyrazol-1-yl]adenosine](https://images-eureka.patsnap.com/patent_img/e49ee01a-c3fa-40ac-b011-1d96cff35528/US09441006-20160913-D00003.PNG)