Novel technetium-99m-labeled higher fatty acid derivative
A high-grade fatty acid, -99m technology, applied in the field of high-grade fatty acid derivatives, can solve the problems of unfavorable myocardial imaging, slow blood clearance, slow metabolism, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074]
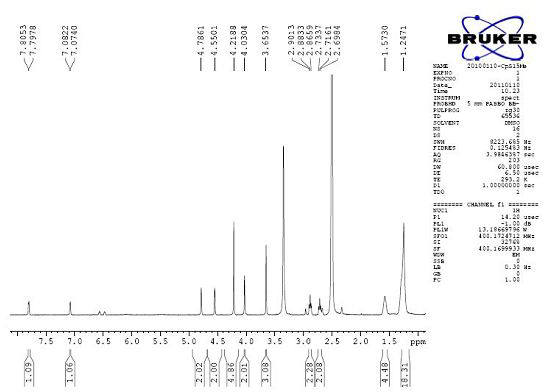
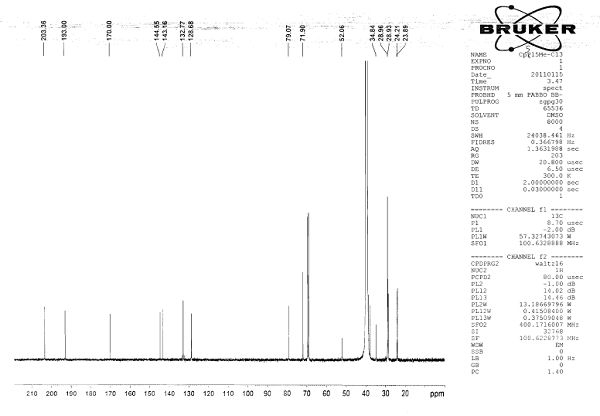
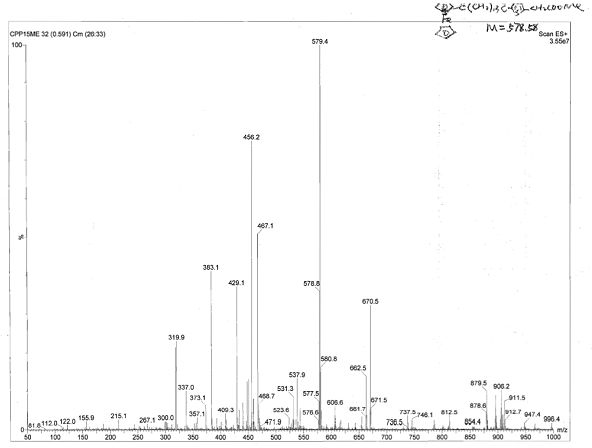
[0075] Compound 1: 15-cyclopentadienyltricarbonyltechnetium-15-carbonylpentadecanoyl-5-thiophene-2-acetic acid ( 99m Tc-CpTTOPTAA)
[0076] The compound one ( 99m The synthetic route of Tc-CpTTOPTAA) is:
[0077]
[0078] The synthetic steps of described compound one are:
[0079] (1) Under ice bath conditions, dilute 15 mL of freshly distilled SOCl 2 Slowly add it dropwise to 11mmol pentadecanoyl acid. After the dropwise addition, continue to stir for 10 minutes, then raise the temperature to 90°C, and reflux for 12 hours. The following reaction occurs to generate the product pentadecanoyl chloride. After the reaction, the excess SOCl was evaporated 2 , the product is directly reacted in the next step without further purification; step (1) corresponds to the following reaction formula:
[0080]
[0081] (2) Add 15 mL of anhydrous dichloromethane to the product obtained in step (1) to dissolve to obtain a dichloromethane solution of pentadecanoyl chlori...
Embodiment 2
[0099]
[0100] Compound 2: 17-cyclopentadienyltricarbonyltechnetium-17-carbonylheptadecanoyl-5-furan-2-acetic acid ( 99m Tc-CpTTOPFAA)
[0101] The compound two ( 99m The synthetic route of Tc-CpTTOPFAA) is:
[0102]
[0103] The synthetic steps of described compound two are:
[0104] (1) Under the condition of ice bath, put 15mL freshly distilled SOCl 2 Slowly add it dropwise to 11 mmol of heptadecanoic acid. After the dropwise addition, continue to stir for 10 minutes, then raise the temperature to 80-90°C, and reflux for 6-12 hours. The following reaction occurs to generate the product heptadecanoyl chloride. After the reaction, the excess SOCl was evaporated 2 , the product was directly reacted in the next step without further purification. Step (1) corresponds to the following reaction:
[0105]
[0106] (2) Add 15mL of anhydrous dichloromethane to the product obtained in step (1) to dissolve to obtain a dichloromethane solution of seventeen-acioyl chlori...
Embodiment 3
[0115]
[0116] Compound 3: 17-cyclopentadienyltricarbonyltechnetium-17-carbonylheptadecanoyl-5-(N-methyl)pyrrole-2-acetic acid
[0117] ( 99m Tc-CpTTOPMPAA)
[0118] The compound three ( 99m The synthetic route of Tc-CpTTOPMPAA) is:
[0119]
[0120] The synthetic steps of described compound three are:
[0121] (1) Under the condition of ice bath, put 15mL freshly distilled SOCl 2 Slowly add dropwise to 11mmol heptadecanoic acid, after the dropwise addition, continue to stir for 10min, then raise the temperature to 80-95°C, reflux for 6-12 hours, the following reaction occurs, and the product heptadecanoyl chloride is generated; after the reaction, Evaporate excess SOCl 2 , the product is directly reacted in the next step without further purification; step (1) corresponds to the following reaction formula:
[0122]
[0123] (2) Add 15mL of anhydrous dichloromethane to the product obtained in step (1) to dissolve to obtain a dichloromethane solution of seventeen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com