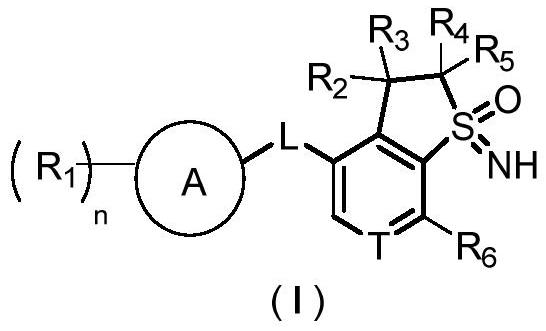
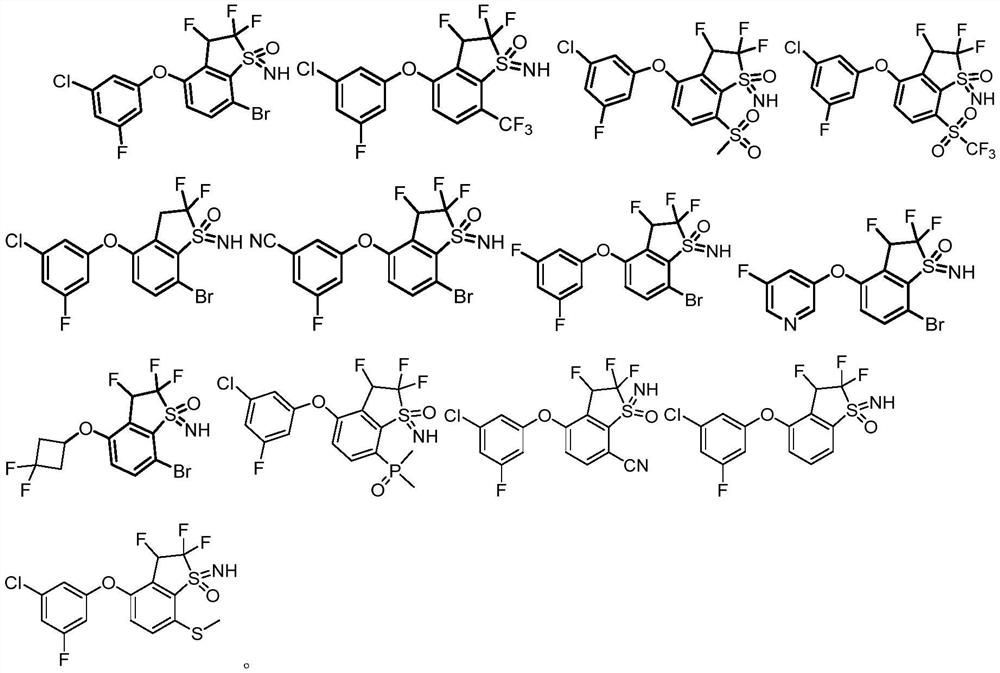
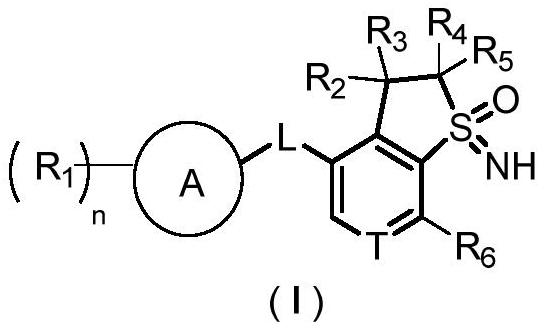
Sulfone imine compound, and preparation method and application thereof
A compound, a technology, applied in organic chemistry, drug combinations, pharmaceutical formulations, etc., can solve problems such as poor prognosis, and achieve the effects of improving PK properties, reducing glucuronidation levels, and improving inhibitory effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1: Compound 1
[0077]
[0078] synthetic route:
[0079]
[0080] Step 1: Preparation of Compound 1-2
[0081] Compound 1-1 (50.0g, 211.0mmol) was dissolved in 800mL DMF, and Na 2 CO 3 (45.0 g, 424.0 mmol). Under nitrogen protection, CH was added dropwise at room temperature 3 I (33.0 g, 232.0 mmol). The reaction solution was stirred at room temperature for 16 h. TLC showed the reaction was complete. Add 1500 mL of water, extract three times with 3000 mL of ethyl acetate, combine the organic phases, wash with water and saturated brine, and dry over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to obtain compound 1-2. 1 H NMR (400MHz, Chloroform-d) δ = 7.63 (ddd, J = 9.0, 7.5, 5.6Hz, 1H), 6.90 (td, J = 8.9, 1.7Hz, 1H), 3.96 (s, 3H).
[0082] Step 2: Preparation of Compound 1-3
[0083] Compound 1-2 (55.0 g, 210.0 mmol) and sodium methyl mercaptide (18.5 g, 264.0 mmol) were added to 600 mL DMA, and stirred for 16 h at 7...
Embodiment 2
[0102] Example 2: Compound 2
[0103]
[0104] synthetic route:
[0105]
[0106] synthetic route:
[0107] Step 1: Preparation of Compound 2
[0108] Compound 1 (6.0 mg, 0.013 mmol) was dissolved in 0.2 mL DMF, and cuprous cyanide (3.6 mg, 0.040 mmol) was added. It was sealed under nitrogen atmosphere, and the reaction solution was heated to 170° C. with microwave and stirred for 1 h. LCMS showed the reaction was complete. 2 mL of water was added, extracted four times with 8 mL of dichloromethane, the combined organic phases were washed with water and saturated brine, and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated and purified by preparative HPLC to obtain compound 2. 1 H NMR (400MHz, Chloroform-d) δ=7.94(dd,J=8.8,1.6Hz,1H),7.20-7.11(m,2H),6.98-6.96(m,1H),6.84-6.78(m,1H ), 6.12 (dd, J=50.8, 9.6Hz, 1H), 4.18 (brs, 1H), LCMS m / z=390.8[M+1] + .
Embodiment 3
[0109] Embodiment 3: compound 3, compound 4
[0110]
[0111] synthetic route:
[0112] Step 1: Preparation of Compound 3 and Compound 4
[0113] Compound 1 (20.0 mg, 0.045 mmol) was dissolved in 1 mL of 1,4-dioxane, and dimethylphosphine oxide (3.5 mg, 0.045 mmol), triethylamine (9.1 mg, 0.090 mmol) and Pd( dppf)Cl 2 (3.3 mg, 0.004 mmol). Under the protection of nitrogen, the reaction solution was stirred at 90° C. for 16 h. LCMS showed the reaction was complete. Add 3 mL of water, extract three times with 9 mL of dichloromethane, wash the combined organic phase with water and saturated brine, and dry over anhydrous sodium sulfate. After filtration, the filtrate was concentrated and purified by preparative HPLC to obtain compound 3 and compound 4.
[0114] Compound 3: 1 H NMR (400MHz, Chloroform-d)δ=8.17-7.98(m,1H),7.20-7.13(m,1H),7.09-7.06(m,1H),6.97-6.94(m,1H),6.80(dt ,J=8.8,2.4Hz,1H),6.27-5.98(m,1H),4.87-4.58(m,1H),2.10-1.89(m,6H),LCMS m / z=442.0[M+1] + .
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com