Regadenoson crystal form and preparation method thereof
A technology of regadesone and crystal form, applied in the field of medicinal chemistry, can solve problems such as harshness, compound decomposition, difficulty in repeating and amplifying
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Preparation of Regardson crystalline form E
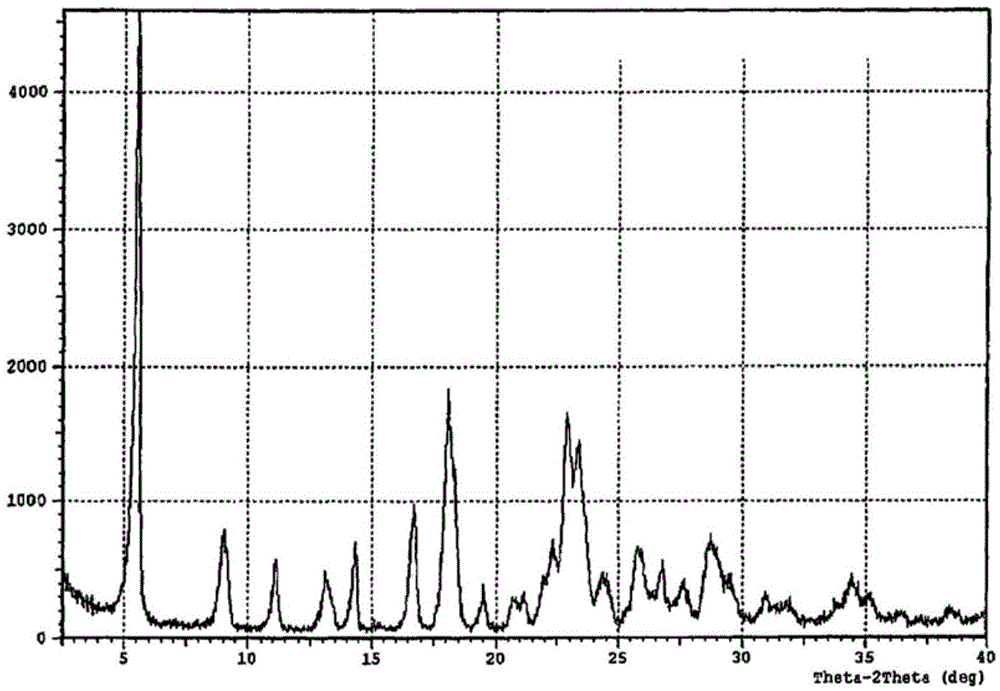
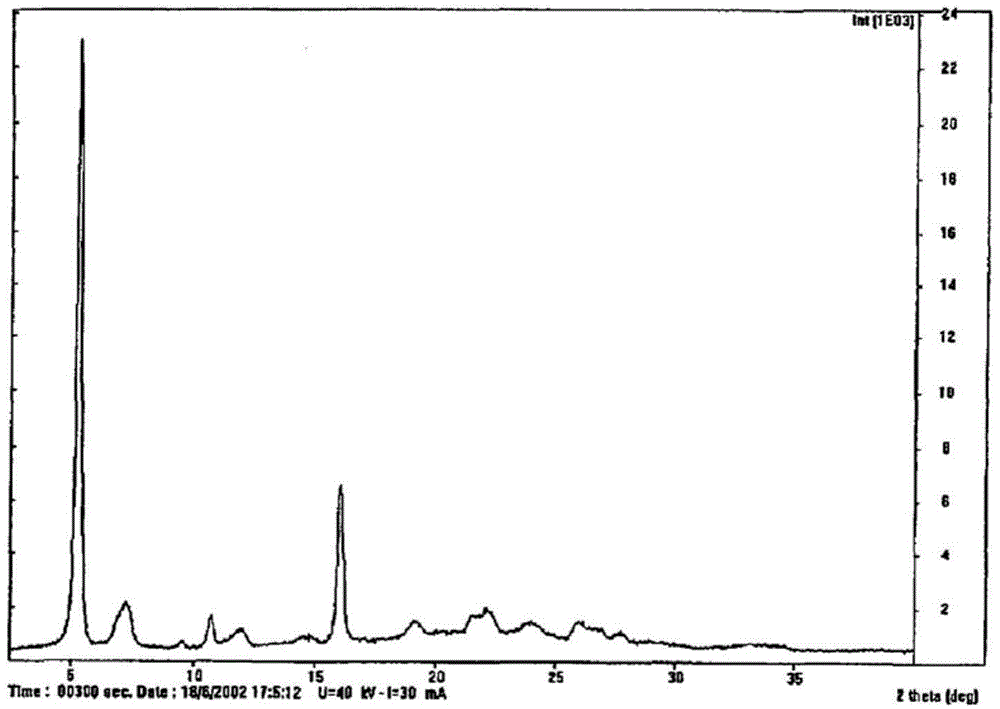
[0040] Dissolve 1 g of regadepine in 10 mL of DMF, add 10 mL of dichloromethane, and concentrate under reduced pressure at 50° C. to obtain 1 g of regadepine crystal form E with a content of 92%. The X-ray powder diffraction pattern and the infrared spectrogram of this crystal form are respectively as follows Figure 5 , Figure 6 shown.
Embodiment 2
[0041] Embodiment 2: Preparation of Regardson crystalline form E
[0042] Dissolve 1 g of regadepine in 10 mL of DMF, add 10 mL of tetrahydrofuran, and concentrate under reduced pressure at 50° C. to obtain 1 g of regadepine crystal form E with a content of 91%. The X-ray powder diffraction and infrared spectrum results of this crystal form are consistent with Example 1.
Embodiment 3
[0043] Embodiment 3: Preparation of Regardson crystalline form E
[0044] Dissolve 1 g of regadepine in 10 mL of DMF, add 5 mL of ethanol, and concentrate under reduced pressure at 50° C. to obtain 1 g of regadepine crystal form E with a content of 91%. The X-ray powder diffraction result and infrared spectrum of this crystal form are consistent with Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com