Myocardial perfusion imaging methods and compositions
a technology of myocardial perfusion and imaging methods, applied in the field of myocardial perfusion imaging methods, can solve the problems of limited use, limited usefulness of treatment with this compound, and many patients' inability to exercise at the level necessary to provide sufficient blood flow, etc., and achieve the effect of facilitating myocardial imaging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Three different aqueous pharmaceutical CVT-3146 formulations were used in the Examples below.
Examples 1-7 employed aqueous pharmaceutical compositions (a) and (b) below. Pharmaceutical compositions (a) and (b) were aseptically filled into 10 mL type I glass vials. (a) An aqueous pharmaceutical composition consisting of 200 micrograms / mL of CVT-3146 in 0.5 (w:v) methylboronic acid buffered with sodium bicarbonate to a pH of 9.3. (b) An aqueous pharmaceutical composition including 200 micrograms / mL of CVT-3146, 0.1% (w:v) methylboronic acid, 50 mM sodium bicarbonate buffer adjusted to pH 9.3 with the addition of 0.55% (w:v) NaCl to make an isotonic pharmaceutical composition.
Example 8 employed a pharmaceutical composition consisting of an aqueous composition of 100 micrograms / mL CVT-3146 in 15% (w:v) propylene glycol and 100 mM phosphate buffer at pH 7 with 0.1% EDTA. The formulation was stored in type I glass vials at 5 mL per vial.
example 1
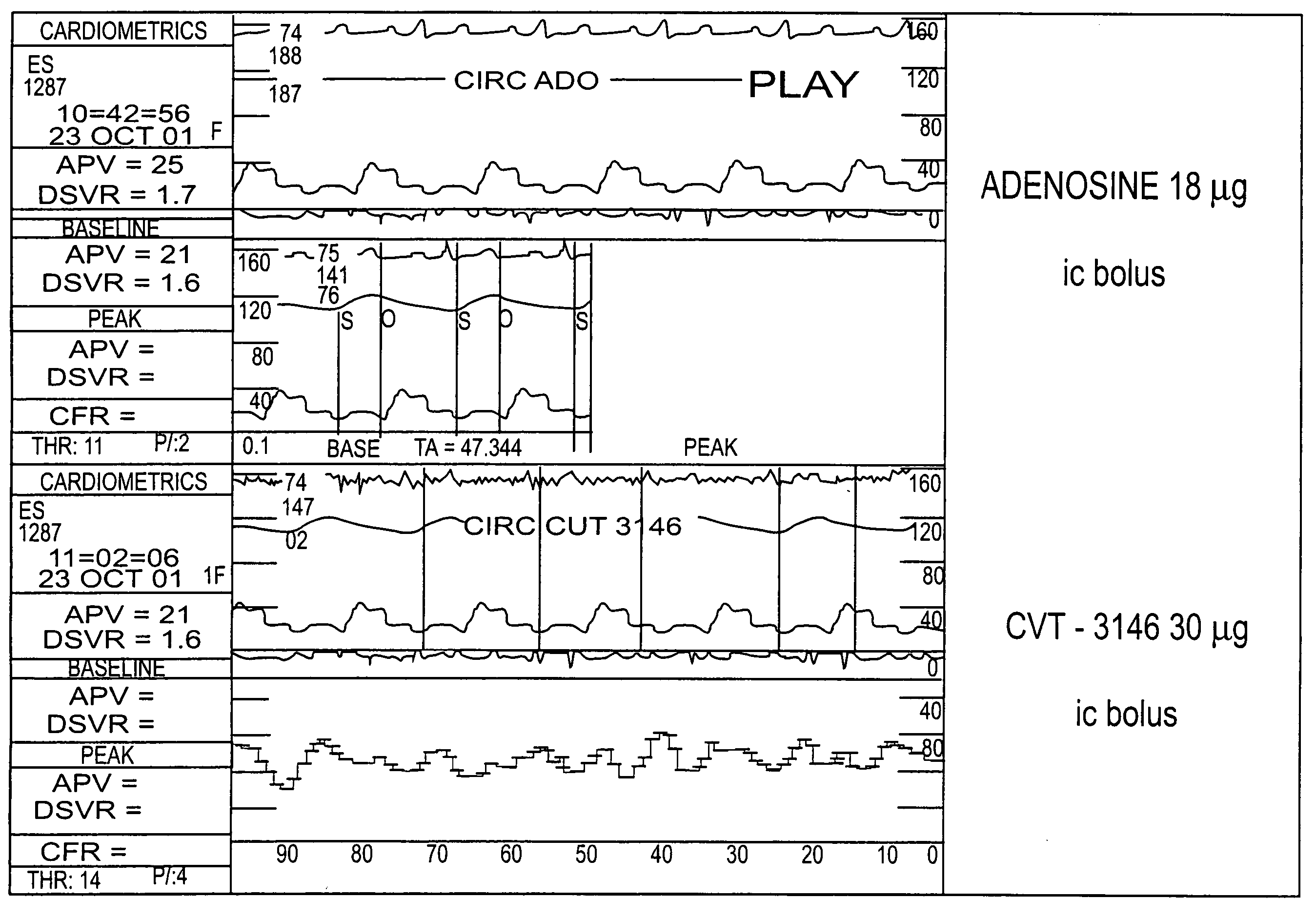
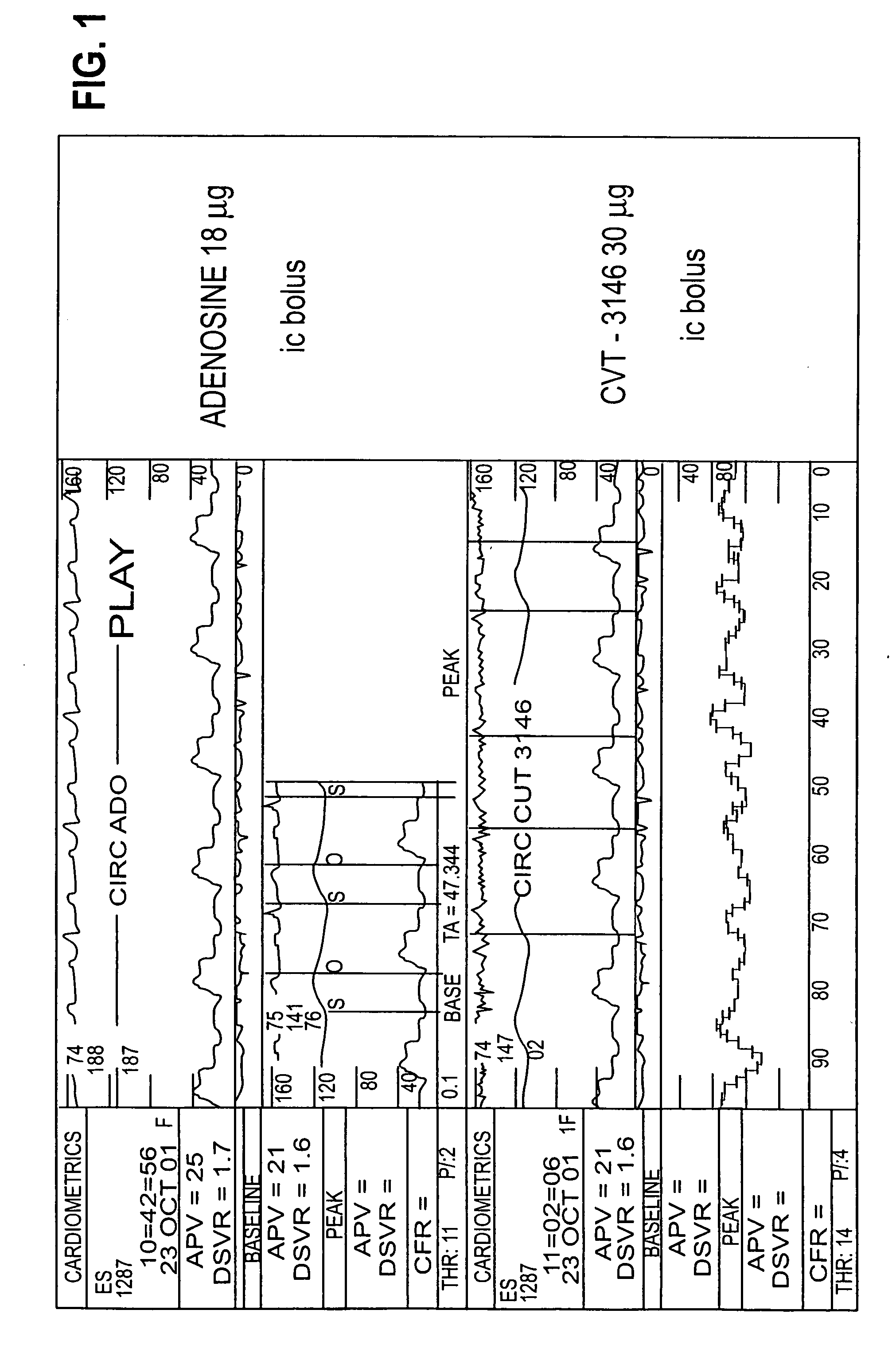
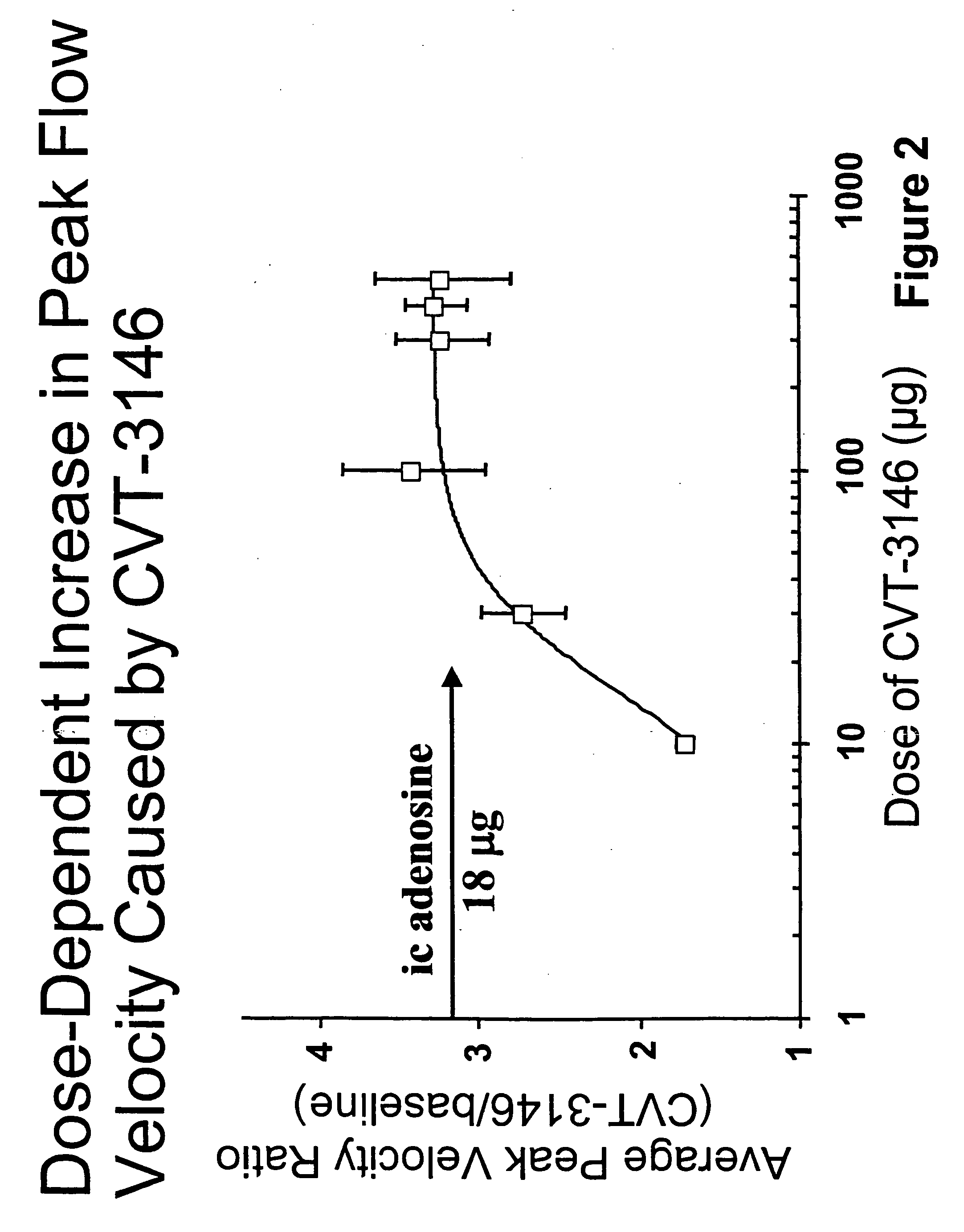
BACKGROUND: CVT-3146 (CVT), with an initial half-life of 3 minutes with a rapid onset and offset of action, is >100-fold more potent than adenosine (Ado) in increasing coronary blood flow velocity (CBFv) in awake dogs. The purpose of this open label study was to determine the magnitude and duration of effect of CVT-3146 (10-500 μg) on CBFv in humans.
METHODS: Patients undergoing a clinically indicated coronary catheterization with no more than a 70% stenosis in any coronary artery and no more than a 50% stenosis of the study vessel had CBFv determined by Doppler flow wire. Study subject were selected after measuring baseline and peak CBFv after an intracoronary (IC) injection of 18 μg of Ado. Twenty-three patients, who were identified as meeting the study criteria of having a peak to baseline CBFv ratio of ≧2.5 in response to Adenosine, received a rapid (≦10 sec) peripheral IV bolus of CVT-3146; Doppler signals were stable and interpretable over the time-course of the increase in ...
example 2
This example is a study performed to determine the range of dosages over which the selective A2A receptor agonist, CVT-3146 can be administered and be effective as a coronary vasodilator.
The study included patients undergoing a clinically indicated coronary catheterization with no more than a 70% stenosis in any coronary artery and no more than a 50% stenosis of the study vessel had CBFv determined by Doppler flow wire. Study subject were selected after measuring baseline and peak CBFv after an intracoronary (IC) injection of 18 μg of Ado. 36 subjects were identified as meeting the study criteria of having a peak to baseline CBFv ration of ≧2.5 in response to Adenosine, CVT-3146 was administered to the study subjects by IV bolus in less that 10 seconds in amounts ranging from 10 μg to 500 μg.
The effectiveness of both compounds was measured by monitoring coronary flow velocity. Other coronary parameters that were monitored included heart rate and blood pressure. These parameters...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com