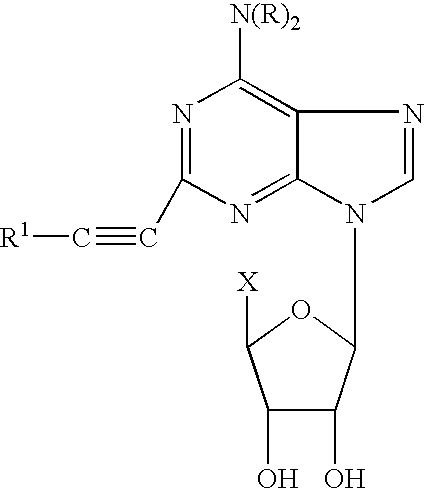
Adenosine derivative formulations for medical imaging
a technology of adenosine derivatives and formulations, applied in the field of adenosine derivative formulations, can solve the problems of many patients absolutely not being able to exercise to any satisfactory level, many adenosine derivatives can be very difficult to solubilize in aqueous media, and many patients cannot achieve satisfactory exercise. the effect of reducing the number of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
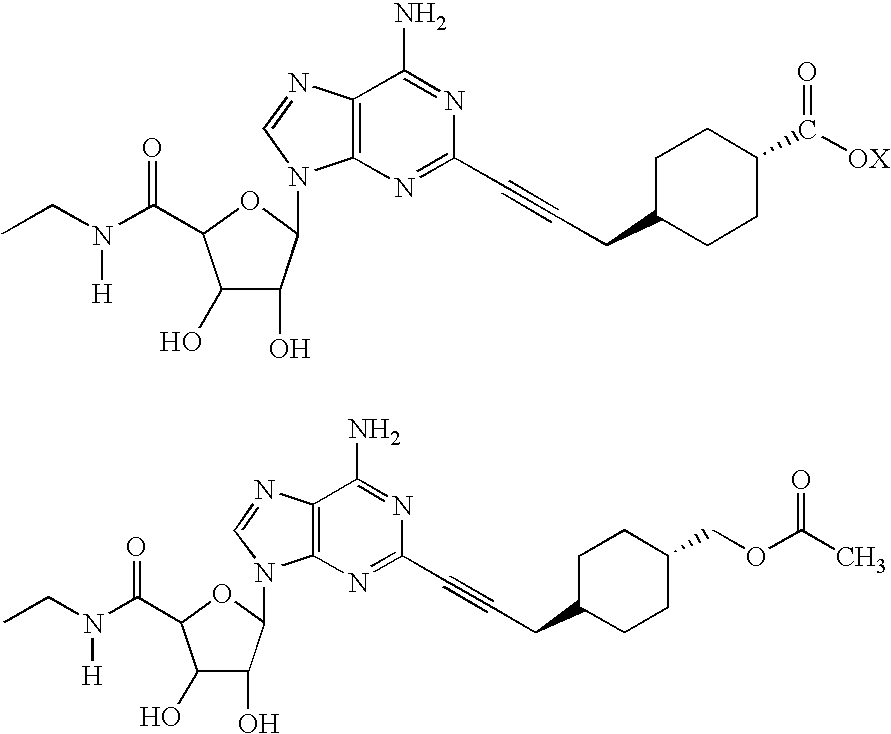
[0043]The following composition was prepared as hereinabove described:
ComponentsAmount or DescriptionMethyl trans-4-[3-[6-amino-9-(N-ethyl-β-D-100 μg / mLribofuranosyluronamide)-9H-purin-2-yl]prop-2-ynyl]cyclohexane carboxylateSodium Citrate Buffer10 mM at pH 4.8 ± 0.2Hydroxypropyl-β-2% by weightCyclodextrin(CAS 128446-35-5)(Hydroxypropyl Betadex[USP])WFI (water for injection)Q.S.HeadspaceAirFill Volume3 mL
[0044]After preparation, the above formulation was aluminum crimp-sealed in a 5 cc tubing vial, and closed with a 20mm serum stopper. Also prepared was a formulation containing 50 μg / mL of active substance, together with 1% by weight of hydroxypropyl-β-cyclodextrin.
example 2
[0045]The present study established the bioequivalency of the pharmacokinetic parameters for one formulation according to a preferred embodiment of the invention versus another formulation in an anesthetized open chest canine model using the same adenosine analog. Five female mongrel dogs, 1.5-2 years of age (11-15 kg) were used. After surgical instrumentation and a stabilization period, each dog received a bolus intravenous injection of adenosine (300 μg / kg) as a reference control followed by four intravenous doses (noncumulative) of Formulation 1 (the invention) and Formulation 2 (a lyophilized formulation) (two 1 μg / kg doses of each formulation). Blood samples were collected at 0 (pre-dose), 1, 3, 5, 7, 10, 15 and 30 minutes post-injection of each dose for determination of plasma levels of the active drug substance adenosine analog and the carboxylic acid metabolite by LC / MS / MS. Pharmacokinetic (PK) analysis included Cmax, area under the time-plasma concentration curve (AUC), cle...
example 3
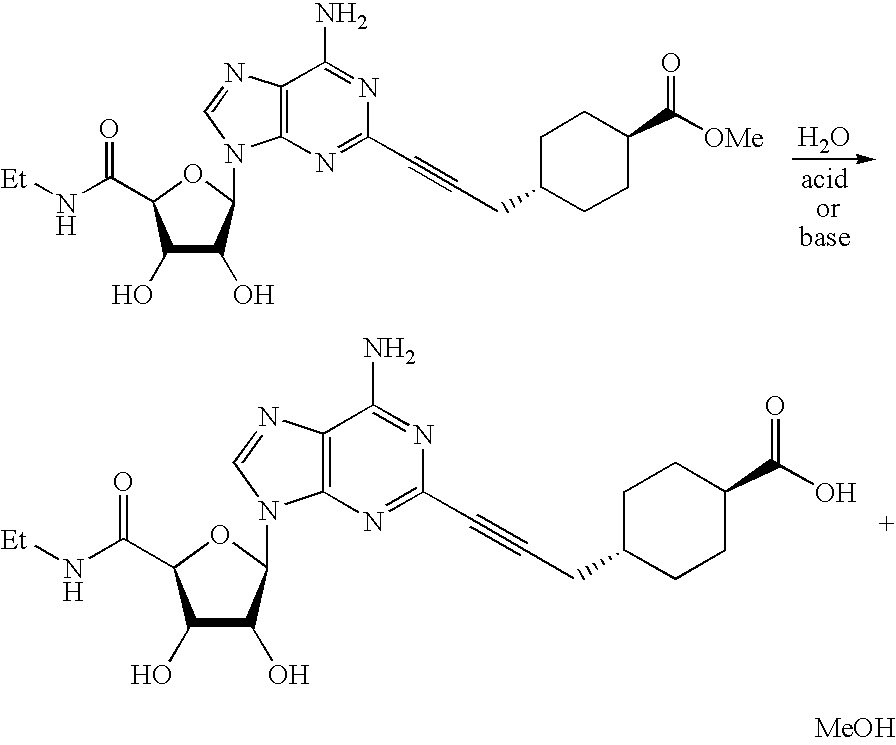
[0049]This example illustrates the comparative stability of adenosine derivative compositions using hydroxypropyl-β-cyclodextrin as part of the invention, versus those containing hydroxyethyl-β-cyclodextrin.
[0050]A solution of methyl trans4-[3-[6-amino-9-(N-ethyl-β-D-ribofuranosyluronamide)-9H-purin-2-yl]prop-2-ynyl]cyclohexane carboxylate at 50 μg / mL was prepared in 10 mM citrate buffer at pH 4.8 with 1% (w / v) of hydroxypropyl-62 -cyclodextrin. A similar solution was prepared except that the 1% (w / v) hydroxypropyl-β-cyclodextrin was replaced with 1% (w / v) hydroxyethyl-β-cyclodextrin. Both solutions were stored at 70° C. for a period of fourteen days. The stability of the adenosine derivative was evaluated over the fourteen day period using chromatography to measure the increase in the primary degradation product, which is generated by hydrolysis of the methyl ester to form the acid by the reaction shown below.
[0051]A plot of the amount of the acid (% w / w relative to the adenosine d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| stable | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com