Method for asymmetrically synthesizing glabridin with optical purity
A technology of optical purity and glabridin, which is applied in organic chemistry methods, organic chemistry, bulk chemical production, etc., can solve the problems of human toxicity and expensive raw materials, and achieve convenient operation, good product yield, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
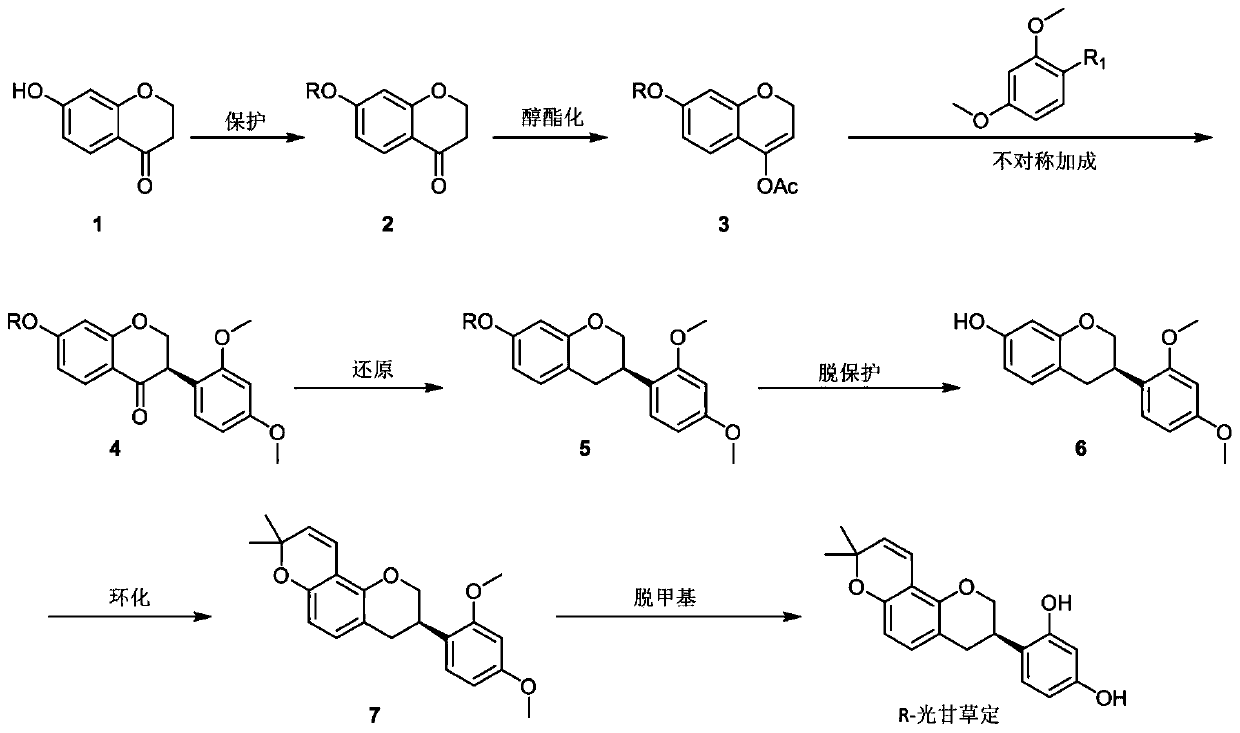
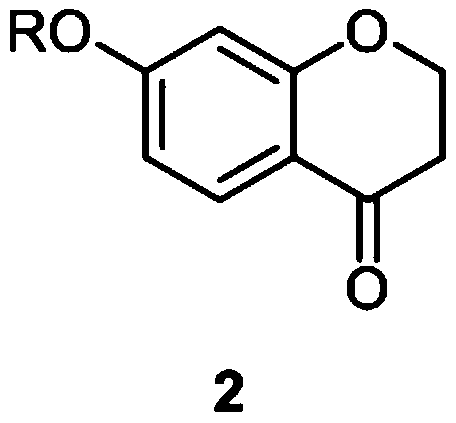
[0047] (1) Synthesis of 7-benzyloxy chroman-4-one (compound 2)
[0048] In a three-neck flask equipped with a thermometer and a condenser, accurately weigh the KOH solid (0.11 mol) and 95% ethanol (40 mL), heat it to full dissolution, and add 7-hydroxychroman-4-one (0.1 mol), The obtained solution was slowly added dropwise to the refluxing benzyl chloride (0.125mol) ethanol solution (20ml), the dropping time was about 1h, after the dropping was completed, the reaction was continued for 1h at the reflux temperature. After the reaction, the solvent was distilled off under reduced pressure, and the residue was recrystallized from ethanol to obtain compound 2 with a yield of 87%.
[0049] 2) Synthesis of 7-benzyloxy-2H-chroman-4-yl acetate (compound 3)
[0050] Put compound 2 (1mmol), propylene acetate (3mmol) and p-toluenesulfonic acid (0.1mmol) in a reaction flask, stir and heat to reflux for 24h, cool to room temperature, distill the solvent under reduced pressure, and dissolve the o...
Embodiment 2
[0062] The synthesis of compounds 3, 4, 5, 6, 7 and R-glycyrrhizin is the same as that of Example 1, except for the synthesis of compound 2.
[0063] Synthesis of compound 2:
[0064] In a three-necked flask equipped with a thermometer and a condenser, accurately weigh out 0.11 mol of NaOH solid and 40 mL of 95% ethanol, heat them to full dissolution, add 7-hydroxychroman-4-one, and slowly drop the resulting solution Add to the 0.125mol benzyl chloride ethanol solution (20ml) that has refluxed, and the dripping time is about 1h. After the dripping is completed, the reaction is continued for 1h at the reflux temperature. After the reaction, the solvent was distilled off under reduced pressure, and the residue was recrystallized from ethanol to obtain compound 2 with a yield of 85%.
Embodiment 3
[0066] The synthesis of compounds 3, 4, 5, 6, 7 and R-glycyrrhizin is the same as that of Example 1, except for the synthesis of compound 2.
[0067] Synthesis of compound 2:
[0068] Compound 1 (0.1 mol) and potassium carbonate (0.12 mol) were added to 100 mL of acetone at room temperature, and then benzyl bromide (0.12 mol) was slowly added, and the reaction was carried out overnight. After the reaction, it was filtered, the solvent was evaporated under reduced pressure, and recrystallized with ethanol to obtain compound 2 with a yield of 88%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap