A kind of staphylococcus aureus enterotoxin a tag peptide and its application
A Staphylococcus gut, golden yellow technology, applied in the direction of peptides, decapeptides, markers used in chemical analysis, etc., can solve the problems of false positives, false negatives, etc., achieve good linearity, ensure accuracy, ensure feasibility and safety sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Create a standard curve:
[0075] Accurately draw 25 μL, 50 μL, 100 μL, 200 μL, 400 μL, and 800 μL of the standard solution of SEA tag peptide with a concentration of 20 nM into 1.5 mL plastic centrifuge tubes, add 100 μL of 20 nM internal standard peptide solution, and then use 0.1% formic acid aqueous solution Make up the volume to 1mL, and prepare a series of standard solutions of tagged peptides with concentrations of 0.5nM, 1.0nM, 2.0nM, 4.0nM, 8.0nM, and 16.0nM, respectively.
[0076] A series of standard solutions of prepared tag peptides were detected by high performance liquid chromatography-mass spectrometry.
[0077] The detection conditions of high performance liquid chromatography in this implementation are: chromatographic column: Acquity UPLC BEH Peptide 300C18 column (2.1×100mm, 1.7μm); column temperature: 40°C; injection volume: 5μL; mobile phase A: 0.1% formic acid- Water, mobile phase B: 0.1% formic acid-acetonitrile; Gradient elution: 0min-1min, 5% ...
Embodiment 2
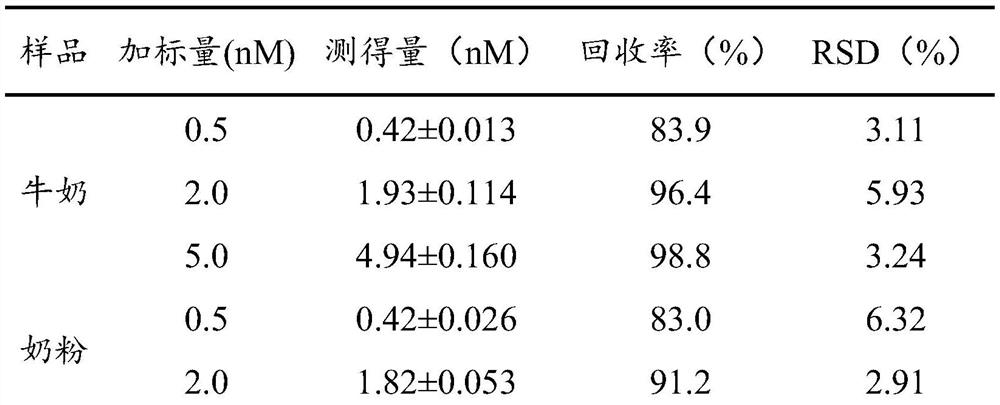
[0081] Determination of SEA content in milk:
[0082] Weigh 25g of milk sample, adjust the pH to 3.8 with hydrochloric acid, and centrifuge at 10000 rpm for 15min at 4°C. Transfer the supernatant to a new centrifuge tube, adjust the pH to about 7.5 with 5M sodium hydroxide solution, and centrifuge at 10,000 rpm for 15 min at 4°C. Remove the supernatant, add the same volume of 20wt% trichloroacetic acid solution, let stand at 4°C for 30min, then centrifuge at 10000 rpm for 10min at 4°C. Discard the supernatant, wash the precipitate with 5 mL of ice ethanol (pre-cooled to 4°C), vortex and mix well, and centrifuge at 10,000 rpm for 10 min at 4°C, discard the supernatant, and repeat this washing operation twice. The washed precipitate was dried under nitrogen flow (temperature below 30° C.). The precipitate was dissolved with 5 mL of 100 mM Tris-HCl (pH 8.5) buffer to obtain a pretreated sample.
[0083] Pipette 200 μL of the pretreated sample, add 100 μL of 20 nM internal stan...
Embodiment 3
[0086] Determination of SEA content in milk powder:
[0087] Reconstitute the milk powder into milk according to the brewing ratio on the manufacturer's package, and weigh 25g of milk after mixing homogeneously. The sample to be tested was prepared according to the same method as in Example 2, and the sample to be tested was detected according to the high performance liquid chromatography-mass spectrometry technology and detection conditions provided in Example 1. Test results: the milk powder provided in this example does not contain Staphylococcus aureus enterotoxin A.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap